b Research Center of Molecular Medicine, Ministry of Education, Key Laboratory of Fujian Molecular Medicine, Key Laboratory of Precision Medicine and Molecular Diagnosis of Fujian Universities, Key Laboratory of Xiamen Marine and Gene Drugs, School of Biomedical Sciences, Huaqiao University, Xiamen 361021, China
Biaryls have attracted considerable attention in the fields of electrochemistry, photochemistry and biochemistry, because they are privileged π-conjugated cores in biologically active molecules, advanced functional materials, ligands, and synthetic intermediates [1]. Over the last decade, metal-catalyzed direct dehydrogenative coupling reactions via dual C–H bond activation have been developed as the most attractive and powerful approaches to biaryls, since the starting materials could be used directly without prior functionalization, and the sole byproduct would only be 2 equiv. of H+ [2]. In 1965, van Helden and Verberg disclosed the first oxidative C—H/C—H coupling of aromatic compounds in the presence of stoichiometric amounts of PdCl2 to synthesize biaryls [3]. Subsequently, in 2006, Lu and co-workers disclosed an intermolecular cross-coupling of simple arenes via C—H activation for synthesis of biaryls employing Pd(OAc)2/TFA/K2S2O8 as the catalytic system [4]. After that, the regioselective palladium-catalyzed homocoupling of indolizines was also demonstrated by You and co-workers in 2009 [5]. Recently, Zhou and Wang reported an efficient oxidative homocoupling of benzene to synthesize biaryls by using O2 as the sole oxidant, in which low catalyst loading (Pd(OAc)2, 0.07 mol%) was required [6]. Also noteworthy is a palladium-catalyzed oxidative homocoupling of 3-arylbenzo[d]-isoxazoles via direct C—H bond activation using benzoisoxazole as a new directing group reported by Ji group simultaneously [7].
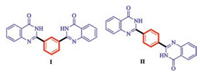
As well-known, quinazolinone has become increasingly valuable framework because of its biological activities, such as antibacterial, antifungal, antimalarial, anticancer, antihypertensive, antitubercular, and anticonvulsant [8]. Logically, biaryls with two quinazolinone units could be potentially used for the development of new drugs or advanced functional materials. For example, compounds Ⅰ and Ⅱ (Fig. 1) show activity against Grampositive and Gram-negative bacteria and yeasts [9].
|
Download:
|
| Fig. 1. Biologically active biaryls containing two quinazolinone units. | |
Recently, our group has developed a series of easy methods for modification of arene via C–H bond activation, in which quinazolinones act as directing groups since there are two N atoms in the molecule [10]. However, to the best of our knowledge, no example was reported on the direct construction of biaryls with two 2-arylquinazolinone units. In our continuing effort to build new molecules through C–H bond activation [11], herein, we present a Pd-catalyzed oxidative homocoupling of 2-arylquinazolinones with O2 as an oxidant using quinazolinone as a directing group (Scheme 1). New well-defined structure possessed two 2-arylquinazolinone units was obtained with high efficiency and atomic economy. This protocol features simple operation, easily available starting materials, high efficiency, environmental friendliness, and tolerance of a wide range of substrates.

|
Download:
|
| Scheme 1. Oxidative homocoupling of 2-arylquinazolinones. | |
Initially, 2-phenylquinazolin-4(3H)-one (1a) was chosen as a model substrate to examine the impact of various parameters on the reaction (Table 1). The results revealed that 2, 20-([1, 10-biphenyl]-2, 20-diyl)bis(quinazolin-4(3H)-one) (2a) was obtained as a main product in 58% yield in DMF at 120 ℃ when Pd(OAc)2 (10 mol%) was used as a catalyst with CuI (0.5 equiv.) under O2 (1 atm) (Table 1, entry 1). Only 25% yield of the target product 2a was achieved when the reaction was carried out under air atmosphere (Table 1, entry 2). No product was obtained under N2 protection (Table 1, entry 3). Palladium salts, such as PdCl2, Pd(PPh3)4 and Pd2(dba)3 were screened. Pd(OAc)2 gave the highest yield (Table 1, entry 1, entries 5–7). The reaction did not occur in the absence of Pd salt (Table 1, entry 4). Moreover, CuBr favors this reaction than other Cu salts (Table 1, entry 1, entries 8–13), and the yield of 2a was increased to 90% in the presence of CuBr. DMF was demonstrated to be better than other solvents, such as DMA, DMSO, DCE and NMP (Table 1, entry 9 vs. entries 14–17). In addition, the yield of 3a was decreased when the reaction temperature and reaction time were changed (Table 1, entries 18–21). However, when other oxidants were used in the reaction, such as oxone and TBHP, only 31% and 19% yields of 2a were achieved, respectively (Table 1, entries 22–23). Based on the results, the optimal reaction conditions were identified as follows: DMF as solvent, at 120 ℃, Pd(OAc)2 (10 mol%) as a catalyst with CuBr (0.5 equiv.) under O2 (Table 1, entry 9).
|
|
Table 1 Optimization of the reaction conditions.a |
With the optimized reaction conditions in hand, the scope of the substrates was examined (Scheme 2). 2-Phenylquinazolin-4 (3H)-one (1a) and its derivatives could react smoothly to give biarlys (2a-2n) in moderate to good yields (55%–90%). F or methy at the ortho-, meta-, and para-position of 2-phenyl in 2-arylquinazolin-4(3H)-ones provided the corresponding products 3b-3g in 71%, 69%, 88%, 76%, 68%, and 85% yields, which indicated that steric effect of 2-aryl group slightly impacted this transformation. Other groups, such as Cl, t-butyl, methoxyl, trifluoromethyl, and nitryl could be well tolerated and gave the corresponding products in satisfactory yields (3h–3L) (56%–85%). These results indicated that the electron density on the moiety of the phenyl did not significantly influence the efficiency of the coupling reaction. 2-(meta-Fluoro)phenylquinazolin-4(3H)-one gave the hindered C2-coupling product 2c, isolated with 69% yield, perhaps due to the ortho-directing effect of the F atom, in which C3 fluoro atom enhances the acidity of C2 proton. While, C6-coupling product 2f at less hindered position was obtained in 68% yield as sole isomer. Moreover, the reaction of 5-fluoro-2-(p-tolyl)quinazolin-4(3H)-one and 6-methoxy-2-(p-tolyl)quinazolin-4(3H)-one proceeded smoothly under the standard reaction conditions to give the corresponding products 2m and 2n in 55% and 63% yields. However, when two different 2-arylquinazolinones were loaded in the reaction, such as 2-phenylquinazolin-4(3H)-one (1a) and 2-(p-tolyl)quinazolin-4(3H)-one (1g), the reaction was complex and gave the mixture of homo- and cross- coupling products.

|
Download:
|
| Scheme 2. Scope of substrates. Reaction conditions: 1a (0.20 mmol), Pd(OAc)2 (10 mol%), CuBr (0.5 equiv.), DMF (2.0 mL), O2 (1 atm). Isolated yields. | |
To confirm which N atom of quinazolinone playing as the directing group, NH-protected quinazolinone was used as the substrate. No target product was obtained when 2-(4-fluorophenyl)-3-phenethylquinazolin-4(3H)-one (1o) was treated under the optimized reaction conditions (Scheme 3). The result suggested that nitrogen (NH) of quinazolinones might act as a directing group to coordinate with Pd(Ⅱ).

|
Download:
|
| Scheme 3. Control experiments. | |
Based on the results obtained and the literatures [12], a plausible reaction mechanism is proposed for the direct homocoupling of 2-phenylquinazolin-4(3H)-one (Scheme 4). Firstly, the dimeric cyclopalladated intermediate A was formed through the coordination of Pd(Ⅱ) with 1a, followed byC—H bond activation/cleavage.Then, the single-core intermediate B was enabled through the second C—H bond activation of another substrate molecule (1a). Finally, intermediate B underwent a subsequent reductive elimination to give the homo-coupled product 2a as well as Pd(0) species, which was oxidated into the active Pd(Ⅱ) species for next catalytic cycle.

|
Download:
|
| Scheme 4. Proposed reaction mechanism. | |
In summary, we have demonstrated a Pd-catalyzed aerobic oxidative homocoupling of 2-arylquinazolinones to synthesize 2, 20-([1, 10-biphenyl]-2, 20-diyl)bis(quinazolin-4(3H)-one), in which quinazolinone acts as a directing group. The well-defined products that possessed two quinazolinone units were obtained with high efficiency and atomic economy. This approach provided a fast pathway for atom/step economical syntheses of useful biaryls. Further study on the applications of this reaction is ongoing in our laboratory.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21572072), 111 Project (No. BC2018061) and Y. Feng thanks the financial support of Scientific Research Foundation of Xiamen Huaxia University (No. HX201807), Outstanding Youth Scientific Research Cultivation Plan in Fujian Province University (No. 201808), the Fujian Education and Scientific Research Project for Young and Middle-aged Teachers (No. JAT190990).
Appendix A. Supplementary dataSupplementary material relatedtothisarticle canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.080.
| [1] |
(a) J. Hassan, M. Sevignon, C. Gozzi, et al., Chem. Rev. 102(2002) 1359-1470; (b) C.S.V. Yeung, M. Dong, Chem. Rev. 111(2011) 1215-129; (c) K. Hirano, M. Miura, Chem. Commun. 48(2012) 10704-10714; (d) S.A. Girard, T. Knauber, C.J. Li, Angew. Chem. Int. Ed. 126(2014) 76-103; (e) Y. Wu, J. Wang, F. Mao, et al., Chem. Asian J. 9(2014) 26-47. |
| [2] |
(a) C.J. Li, Z. Li, Pure Appl. Chem. 78(2006) 935-945; (b) M. Matsushita, K. Kamata, K. Yamaguchi, et al., J. Am. Chem. Soc.127(2005) 6632-6640; (c) X.L. Li, J.B. Hewgley, C.A. Mulrooney, et al., J. Org. Chem. 68(2003) 5500-5511; (d) L. Grigorjeva, O. Daugulis, Org. Lett. 17(2015) 1204-1207; (e) A.J. Paterson, S.St. John-Campbell, M.F. Mahon, et al., Chem. Commun. 51(2015) 12807-12810; (f) K. Masui, H. Ikegami, A. Mori, J. Am. Chem. Soc. 126(2004) 5074-5075; (g) M. Takahashi, K. Masui, H. Sekiguchi, et al., J. Am. Chem. Soc. 128(2006) 10930-10933; (h) R. Li, L. Jiang, W. Lu, Organometallics 25(2006) 5973-5975; (i) K. Fagnou, Science 316(2007) 1172-1175; (j) T.A. Dwight, N.R. Rue, D. Charyk, et al., Org. Lett. 9(2007) 3137-3139; (k) X. Qin, D. Sun, Q. You, et al., Org. Lett. 17(2015) 1762-1765; (l) Y. Yang, J. Lan, J. You, Chem. Rev. 117(2017) 8787-8863; (m) J. Wencel-Delord, C. Nimphius, H. Wang, et al., Angew. Chem. Int. Ed. 51(2012) 13001-13005; (o) X. Han, P. Lin, Q. Li, Chin. Chem. Lett. 30(2019) 1495-1502; (p) S. Yuan, S. Wang, M. Zhao, et al., Chin. Chem. Lett. 31(2020) 349-352; (q) Q. Huang, L. Zhu, D. Yi, X. Zhao, W. Wei, Chin. Chem. Lett. 31(2020) 373-376; (r) X. Zhang, S. Dong, Q. Ding, X. Fan, G. Zhang, Chin. Chem. Lett. 30(2019) 375-378. |
| [3] |
R. van Helden, G. Verberg, Recl. Trav. Chim. Pays-Bas 84 (1965) 1263-1273. |
| [4] |
R. Li, L. Jiang, W. Lu, Organometallics 25 (2006) 5973-5975. DOI:10.1021/om060889d |
| [5] |
J.B. Xia, X.Q. Wang, S.L. You, J. Org. Chem. 74 (2009) 456-458. DOI:10.1021/jo802227u |
| [6] |
Y. Liu, X. Wang, X. Cai, et al., ChemCatChem 8 (2016) 448-454. DOI:10.1002/cctc.201500951 |
| [7] |
Y. Guo, K.K. Yu, L.H. Xing, et al., Adv. Synth. Catal. 357 (2017) 410-418. |
| [8] |
(a) A.K. Nanda, S. Ganguli, R. Chakraborty, Molecules 12(2007) 2413-2426; (b) J.H. Chan, J.S. Hong, L.F. Kuyper, et al., J. Med. Chem. 38(1995) 3608-3616; (c) H.Kikuchi, K. Yamamoto, S. Horoiwa, etal., J.Med. Chem.49(2006)4698-4706; (d) Y. Takase, T. Saeki, N. Watanabe, et al., J. Med. Chem. 37(1994) 2106-2111; (e) M. Dupuy, F. Pinguet, O. Chavignon, et al., Chem. Pharm. Bull. 49(2001) 1061-1065; (f) P.M. Chandrika, T. Yakaiah, A.R.R. Rao, et al., Eur. J. Med. Chem. 43(2008) 846-852; (g) M.H. Yen, J.R. Sheu, I.H. Peng, et al., J. Pharm. Pharmacol. 48(1996) 90-95; (h) J. Kunes, J. Bazant, M. Pour, et al., Farmaco 55(2000) 725-729; (i) K. Waisser, J. Gregor, H. Dostal, et al., Farmaco 56(2001) 803-807; (j) A. Archana, V.K. Shrivastava, R. Chandra, et al., Indian J. Chem. 41B (2002) 2371-2375. |
| [9] |
S.A. Shiba, A.A. El-Khamry, M.E. Shaban, et al., Pharmazie 52 (1997) 189-194. |
| [10] |
(a) Y. Feng, N. Tian, Y. Li, et al., Org. Lett. 19(2017) 1658-1661; (b) Y. Feng, Y. Li, Y. Yu, L. Wang, X. Cui, RSC Adv. 8(2018) 8450-8454; (c) Y. Feng, Z. Zhang, Q. Fu, et al., Chin. Chem. Lett. 31(2020) 58-60; (d) Y. Feng, Y. Liu, Q. Fu, et al., Chin. Chem. Lett. 31(2020) 733-736; (e) W. Fu, L. Wang, Z. Shi, et al., Chem. Commun. 56(2020) 560-563; (f) Y. Wu, C. Pi, X. Cui, et al., Org. Lett. 22(2020) 361-364; (g) J. Ren, X. Yan, X. Cui, et al., Green Chem. 22(2020) 265-269; (h) X. Mi, Y. Kong, J. Zhang, et al., Chin. Chem. Lett. 30(2019) 2295-2298; (i) Y. He, C. Pi, Y. Wu, et al., Chin. Chem. Lett. 31(2020) 396-400. |
| [11] |
(a) Y. Li, Y. Feng, L. Xu, et al., Org. Lett. 18(2016) 4924-4927; (b) Y. Li, M. Gao, L. Wang, et al., Org. Biomol. Chem. 14(2016) 8428-8432; (c) M. Gao, Y. Li, L. Xie, et al., Chem. Commun. 52(2016) 2846-2849; (d) M. Wei, L. Wang, X. Cui, Chin. Chem. Lett. 26(2015) 1336-1340; (e) L. Wang, Z. Yang, M. Yang, et al., Org. Biomol. Chem. 15(2017) 8302-8307. |
| [12] |
(a) K.L. Hull, E.L. Lanni, M.S. Sanford, J. Am. Chem. Soc. 128(2006) 14047-14049; (b) S.R. Whitfield, M.S. Sanford, J. Am. Chem. Soc. 129(2007) 15142-15143; (c) J.M. Racowski, A.R. Dick, M.S. Sanford, J. Am. Chem. Soc. 131(2009) 10974-10983; (d) H. Batchu, S. Bhattacharyya, R. Kant, et al., J. Org. Chem. 80(2015) 7360-7374; (e) L. Hull, S. Sanford, J. Am. Chem. Soc. 131(2009) 9651-9653. |
 2020, Vol. 31
2020, Vol. 31 


