b School of Life Sciences and Chemistry, Hunan University of Technology, Zhuzhou 412007, China;
c Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China
Regarding the need for acquiring small-molecule drugs employing energy-saving and eco-friendly protocols to efficiently synthesize organic compounds, the chemists have devoted plenty of efforts. Over the past years, the ultrasound-assisted synthetic technique has been demonstrated as an environmentally friendly and versatile tool in green and sustainable chemistry. Compared with the traditional mechanical agitation, ultrasonic irradiation has the advantages of elevated reaction rate, low reaction temperature, reduced energy consumption, minimized side reactions, and enhanced selectivity and yield. This is because the ultrasonic effect can produce very high temperature and pressure in the local area of a bubble, and promote the mass transfer and turbulence in the liquid. This unique property has a decisive impact on the chemical reaction kinetics through energy dissipation [1]. On the other hand, the solvent-free synthetic reactions not only reduce the amount of toxic organic solvent waste, but also simplify the reaction mixtures and experimental procedure. Unfortunately, in neat reactions, the solvent-free conditions and high concentrations of raw materials tend to lead to a large number of by-products, which require cumbersome and expensive chromatographic purification process. This issue has been overcome by employing ultrasonic irradiation, which allows conducting solvent-free synthetic reactions, combining the advantages of ultrasonic effect and neat reactions [2].
In chemical industry and parallel methodological chemical sciences, the adoption of alkenes with simple structures as building blocks for the construction of complex molecular architectures represents a special advantageous strategy because alkenes are abundant with low cost [3]. Among the many routine operations conducted with alkenes, cycloaddition reactions of olefinic feedstocks to construct nitrogen heterocycles arguably constitute one of the most paramount classes of chemical transformations [4]. On the other hand, pyrrolecarbonitriles are a significant class of nitrogen heterocycles [5] due to their diverse biological activities and specific physical properties. They also have frequently been employed as core structures for the development of complex molecules and pharmaceutical reagents. Compared with the well-established synthetic methods for pyrroles [6], the direct preparation of substituted pyrrole-2-carbonitriles from the inexpensive and readily available simple alkenes is rarely reported. So far, only one method is applicable for the direct synthesis of substituted pyrrolecarbonitriles through multicomponent tandem reaction of alkenes [7], TMSCN and N, N-disubstituted formamide, as reported by Wang and colleagues, in which the 1, 3-disubstituted pyrrole-2-carbonitriles were efficiently achieved (Scheme 1a) [8]. However, only4 examples of tri-substitutedpyrrolecarbonitrileswere obtained with poor to moderate yields in the presence of Cu(OTf)2 as the catalyst and DDQ as the oxidant in n-heptane/Et2NCOMe mixed solvents at 80 ℃ under argon atmosphere. Furthermore, to the best of our knowledge, there are no examples describing the direct construction of fully substituted pyrrolecarbonitriles. It has been acknowledged that the study of structure-activity relationship needs a series of lead compounds with substituents linked to core structures. Therefore, the development of novel protocol for the efficient construction of tri- and tetra-substituted pyrrolecarbonitriles from inexpensive alkenes under metal- and solvent-free conditions is a critical task of in organic synthesis and pharmaceutical research.

|
Download:
|
| Scheme 1. Synthesis of trisubstituted pyrrolecarbonitriles. | |
The control of a reagent that can play dual roles in a chemical reaction to simplify the reaction conditions and reduce chemical waste is a highly attractive concept, but represents a significant synthetic challenge [9]. Molecular iodine is widely utilized as a catalyst and oxidant in organic synthesis due to its costeffectiveness, excellent functional-group tolerance, and environmental friendliness [10]. As part of our continuous efforts on the green synthesis [11], we herein describe an energy-efficient and eco-friendly method for the synthesis of tri- and tetra-substituted pyrrolecarbonitriles through ultrasound-assisted multicomponent tandem reaction of alkenes, TMSCN and N, N-disubstituted formamide in the presence of molecular iodine as the catalyst and oxidant under solvent-free and mild conditions (Scheme 1b).
We initiated our study utilizing styrene (1a), TMSCN (2) and N, N-diethylacetamide (3a) as the model substrates. The reaction of 1a with equivalent amount of 2 and 3a in the presence of NaI under solvent-free conditions yielded 67% of 1-ethyl-5-methyl-3-phenyl-1H-pyrrole-2-carbonitrile (4aa) at 90 ℃ for 24 h (Table 1, entry 1). Subsequently, various iodine reagents were examined (entries 2-5) and the results revealed that molecular iodine to be the optimum promoter (entry 5) for the multicomponent reactions. Varying the temperature did not improve the reaction efficiency (entries 6 and 7). To our delight, changing the traditional heating to ultrasonic radiation (40 kHz/30 W) resulted in the formation of 4aa in a quantitative yield (entry 8). Reducing the ultrasonic power from 30 W to 20 W brought a distinct decrease in the yield, even with a prolonged reaction time (entry 9). Lowering the promoter loading unsurprisingly led to a lower yield of 4aa (entry 10). Control experiment revealed that the iodine reagent was essential for the transformation to occur (entry 11). If TMSCN was instead of TMSN3 or TMSCF3, the multicomponent tandem reaction was not observed.
|
|
Table 1 Optimization of reaction conditionsa. |
To explore the generality of the multicomponent reaction, various styrenes and N, N-disubstituted formamides were studied under the optimal reaction conditions (Table 1, entry 8). To our delight, the present ultrasound-assisted reaction was suitable for a broad range of styrenes (Scheme 2). All the substituted styrene substrates, no matter the para-substituents are electron-donating, electron-neutral or electron-withdrawing, reacted well to deliver the target products in good to excellent yields (4aa-4na). Moreover, a series of meta- and ortho-substituted styrenes were well-tolerated and provided the desired products in 77%-88% yields (4oa-4ra). Given that 1, 2-divinylbenzene contains two potential alkenyl groups, we were pleased to find that only monoaddition product (4sa) was generated in excellent yield. Polycyclic aromatic substituted ethylene (1t) and various internal alkenes (1u-1x) could participate in this transformation efficiently, giving the desired products in good yields. The present protocol would be applicable to aliphatic and benzylic ethylene, affording the corresponding products (4yc-4zc) with good yields. Gratifyingly, a range of cycloalkenes (1A-1D) were all well-tolerated in the current transformation, which significantly expanded the reaction scope. No reaction occurred when propylene was used as the substrate, even after 1 h of ultrasonic irradiation. Finally, the present method could also be successfully extended to various N, N-disubstituted formamides such as N, N-dimethylformamide, N, N-dipropylformamide, N, N-dibutylformamide and piperidine-1-car-baldehyde, affording the target products (4ab-4ae) with good to excellent yields. When N, N-dibenzylformamide was subjected to the reaction condition, only a complicated reaction mixture of unidentified products was detected.

|
Download:
|
| Scheme 2. Reaction scope. Conditions: 1 (0.3 mmol), 2 (0.3 mmol), 3 (0.3 mmol), I2 (0.3 mmol), USI, 40 kHz/30 W, 40 min. Isolated yields were reported. | |
To shed light on the reaction mechanism, some control experiments were performed (Scheme 3). Given that the dihydropyrrolecarbonitrile was formed in Cu-catalyzed oxidative synthesis of 1, 3-disubstituted pyrrolecarbonitrile, we postulated that 4, 5-dihydro-1H-pyrrole-2-carbonitrile (5aa) might be a key intermediate of the present reaction. When 5aa was subjected to the standard conditions, the direct cycloaddition reaction proceeded smoothly to deliver 4aa in excellent yield (Scheme 3a). In addition, under the optimal conditions, the cycloaddition reaction of styrene with 2-(diethylamino)malononitrile (6a) [12] provided only a trace amount of 4aa. Therefore, the nucleophilic addition of in situ formed 6a can be ruled out as the main reaction pathway (Scheme 3b). Performing the reaction under nitrogen atmosphere produced a slightly lower yield of 4aa.
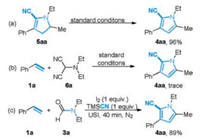
|
Download:
|
| Scheme 3. Control experiments. | |
Based on the experimental observations described above and the reported literature [8], a plausible reaction mechanism for the synthesis of trisubstituted pyrrolecarbonitriles is illustrated in Scheme 4. At first, the ultrasound-assisted molecular iodine catalyzed cyanation of N, N-disubstituted formamide 3 with TMSCN 2 could provide the azomethine ylide intermediate A and resonating with energetically favorable intermediate B. Subsequently, the intermediate B underwent regioselective [3 + 2] cycloaddition to styrene 1 followed by in-situ dehydrogenation to form the dihydropyrrolecarbonitrile intermediate 5. Finally, the oxidative dehydrogenation and aromatization of intermediate 5 would afford the target product 4.
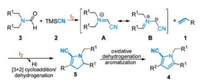
|
Download:
|
| Scheme 4. Plausible reaction mechanism. | |
In conclusion, we have established an energy-saving and ecofriendly method for the preparation of various tri- and tetrasubstituted pyrrolecarbonitriles through ultrasound-assisted tandem reaction of readily available alkenes, TMSCN and N, N-disubstituted formamides within 40 min under metal-, solventfree and mild conditions. The present reaction efficiently proceeded (77%-97%) with high region- and chemoselectivity and a wide range of substrate scope (aromatic, aliphatic and cycloalkenes) as well as excellent functional group compatibility. Moreover, the dual role of iodine (catalyst and oxidant) notably simplified the reaction conditions and reduced the chemical waste generated. Compared with the conventional heating-conditions, the usage of ultrasonic irradiation not only improved the reaction efficiency and rate but also reduced the side-reactions. This protocol will be of choice for the construction of a variety of polysubstituted pyrrolecarbonitriles, some of which are difficult to prepare through conventional approaches.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe are grateful for financial support from the Hunan Provincial Natural Science Foundation of China (Nos. 2018JJ3215 and 2019JJ20008), the Research Foundation of Education Bureau of Hunan Province (No. 18C0147) and Double first-class construction project of Hunan Agricultural University (No. SYL2019064).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.017.
| [1] |
G. Chatel, R.S. Varma, Green Chem. 21 (2019) 6043-6050. |
| [2] |
(a) S.G. Pharande, A.R. Corrales Escobosa, R. Gamez-Montano, Green Chem.19(2017) 1259-1262; (b) C. Wu, L.H. Lu, A.Z. Peng, et al., Green Chem. 20(2018) 3683-3688; (c) L.H. Lu, S.J. Zhou, M. Sun, et al., ACS Sustain. Chem. Eng. 7(2019) 1574-1579. |
| [3] |
(a) M.H. Huang, W.J. Hao, B. Jiang, Chem. Asian J. 13(2018) 2958-2977; (b) M.H. Xu, K.L. Dai, Y.Q. Tu, et al., Chem. Commun. 54(2018) 7685-7688; (c) S.S. Jiang, Y.C. Wu, S.Z. Luo, et al., Chem. Commun. 55(2019) 12805-12808; (d) K. Sun, B. Luan, Z. Liu, et al., Org. Biomol. Chem. 17(2019) 4208-4211; (e) C. Wan, R.J. Song, J.H. Li, Org. Lett. 21(2019) 2800-2803; (f) H. Ruan, L.G. Meng, L. Zhu, L. Wang, Adv. Synth. Catal. 361(2019) 3217-3222; (g) Q. Liu, F. Liu, H. Yue, et al., Adv. Synth. Catal. 361(2019) 5277-5282; (h) L. Zhao, P. Li, H. Zhang, L. Wang, Org. Chem. Front. 6(2019) 87-93; (i) G.P. Yang, X. Wu, B. Yu, C. Hu, ACS Sustain. Chem. Eng. 7(2019) 3727-3732; (j) X. Wang, Y.F. Han, X.H. Ouyang, R.J. Song, J.H. Li, Chem. Commun. 55(2019) 14637-14640. |
| [4] |
(a) S. Chen, Y. Li, P. Ni, H. Huang, G.J. Deng, Org. Lett. 18(2016) 5384-5387; (b) H. Hu, X. Chen, K. Sun, et al., Org. Chem. Front. 5(2018) 2925-2929; (c) F.L. Zeng, K. Sun, X.L. Chen, et al., Adv. Synth. Catal. 361(2019) 5176-5181; (d) J. Lin, R.J. Song, M. Hu, J.H. Li, Chem. Rec. 19(2019) 440-451; (e) X.C. Liu, K. Sun, Q.Y. Lv, et al., New J. Chem. 43(2019) 12221-12224; (f) L. Zou, P. Li, B. Wang, L. Wang, Green Chem. 21(2019) 3362-3369. |
| [5] |
(a) L. Wang, D. Xiong, L. Jie, C. Yu, X. Cui, Chin. Chem. Lett. 29(2018) 907-910; (b) X. Gong, G. Li, Z. Gan, et al., Asian J. Org. Chem. 8(2019) 1472-1478; (c) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30(2019) 2287-2290; (d) G. Li, Q. Yan, X. Gong, X. Dou, D. Yang, ACS Sustain. Chem. Eng. 7(2019) 14009-14015; (e) Z. Wang, W.M. He, Chin. J. Org. Chem. 39(2019) 3594-3595; (f) L. Wang, Y. Zhang, M. Zhang, et al., Tetrahedron Lett. 60(2019) 1845-1848; (g) W. Huang, J. Xu, C. Liu, Z. Chen, Y. Gu, J. Org. Chem. 84(2019) 2941-2950; (h) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31(2020) 67-70; (i)M. Li, X.Dong, N. Zhang, F. Jérôme, Y.Gu, GreenChem. 21(2019) 4650-4655; (j) Y. Wu, C. Pi, X. Cui, Y. Wu, Org. Lett. 22(2020) 361-364. |
| [6] |
(a) Z. Zhang, W. Zhang, J. Li, et al., J. Org. Chem. 79(2014) 11226-11233; (b) A.H. Zhou, Q. He, C. Shu, et al., Chem. Sci. 6(2015) 1265-1271; (c) C. Shu, Y.H. Wang, C.H. Shen, et al., Org. Lett. 18(2016) 3254-3257; (d) Z.W. Gilbert, R.J. Hue, I.A. Tonks, Nat. Chem. 8(2016) 63-68; (e) T. Li, H. Yan, X. Li, C. Wang, B. Wan, J. Org. Chem. 81(2016) 12031-12037; (f) B.Y. Cheng, Y.N. Wang, T.R. Li, L.Q. Lu, W.J. Xiao, J. Org. Chem. 82(2017) 12134-12140; (g) Y. Liu, X. Yi, X. Luo, C. Xi, J. Org. Chem. 82(2017) 11391-11398; (h) A. Kumar, N. Tadigoppula Ramanand, Green Chem. 19(2017) 5385-5389; (i) Z. Tian, J. Xu, B. Liu, Q. Tan, B. Xu, Org. Lett. 20(2018) 2603-2606; (j) A. Kondoh, A. Iino, S. Ishikawa, T. Aoki, M. Terada, Chem. Eur. J. 24(2018) 15246-15253; (k) L. Li, Q. Chen, X. Xiong, et al., Chin. Chem. Lett. 29(2018) 1893-1896; (l) X.Q. Zhu, H. Yuan, Q. Sun, et al., Green Chem. 20(2018) 4287-4291; (m) G. Cheng, W. Lv, L. Xue, Green Chem. 20(2018) 4414-4417; (n) D. Chen, Y. Shan, J. Li, et al., Org. Lett. 21(2019) 4044-4048; (o) J. Cen, Y. Wu, J. Li, et al., Org. Lett. 21(2019) 2090-2094; (p) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. China Chem. 62(2019) 460-464; (q) W. Huang, S. Chen, Z. Chen, et al., J. Org. Chem. 84(2019) 5655-5666; (r) T. Schitter, S. Stammwitz, P.G. Jones, D.B. Werz, Org. Lett. 21(2019) 9415-9419; (s) Q.W. Gui, X. He, W. Wang, et al., Green Chem. 22(2020) 118-122; (t) G. Vengatesh, M. Sundaravadivelu, S. Muthusubramanian, J. Mol. Struct. 1199(2020) 126980; (u) L. Qi, R. Li, X. Yao, et al., J. Org. Chem. 85(2020) 1097-1108. |
| [7] |
(a) J. Jiang, H. Huang, G.J. Deng, Green Chem. 21(2019) 986-990; (b) S. Liu, K. Chen, W.J. Hao, et al., J. Org. Chem. 84(2019) 1964-1971; (c)Z.Xu, G.J.Deng, F.Zhang, H.Chen, H.Huang, Org.Lett.21(2019)8630-8634; (d) H. Huang, Z. Qu, X. Ji, G.J. Deng, Org. Chem. Front. 6(2019) 1146-1150; (e) X.Y. Qin, L. He, J. Li, et al., Chem. Commun. 55(2019) 3227-3230; (f) S. Ye, X. Li, W. Xie, J. Wu, Asian J. Org. Chem. 8(2019) 893-898; (g) G.H. Li, D.Q. Dong, Q. Deng, S.Q. Yan, Z.L. Wang, Synthesis 51(2019) 3313-3319; (h) J. Xu, W. Huang, R. Bai, et al., Green Chem. 21(2019) 2061-2069. |
| [8] |
X.Q. Mou, Z.L. Xu, L. Xu, et al., Org. Lett. 18 (2016) 4032-4035. DOI:10.1021/acs.orglett.6b01883 |
| [9] |
(a) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30(2019) 2259-2262; (b) K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30(2019) 2304-2308; (c) L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30(2019) 1237-1240; (d) W.M. He, Y.W. Lin, D.H. Yu, Sci. China Chem. 63(2020) 291-293; (e) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30(2019) 2132-2138. |
| [10] |
(a) Z. Shi, L. Wang, X. Cui, Chin. J. Org. Chem. 39(2017) 1596-1612; (b) H. Li, P. Zhou, F. Xie, et al., J. Org. Chem. 83(2018) 13335-13343; (c) S.H. Hao, L.X. Li, D.Q. Dong, Z.L. Wang, Chin. J. Catal. 38(2017) 1664-1667; (d) J. Ren, X. Yan, X. Cui, et al., Green Chem. 22(2020) 265-269; (e) S. Du, C. Pi, T. Wan, Y. Wu, X. Cui, Adv. Synth. Catal. 361(2019) 1766-1770; (f) P. Bao, L. Wang, H. Yue, et al., J. Org. Chem. 84(2019) 2976-2983. |
| [11] |
(a) J.Y. Chen, Y.W. Lin, W.M. He, Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2020.03.034; (b) W.B. He, L.Q. Gao, X.J. Chen, et al., Chin. Chem. Lett. 31(2020) 1895-1898; (c) S. Peng, Y.W. Lin, W.M. He, Chin. J. Org. Chem. 40(2020) 541-542; (d) F.H. Qin, X.J. Huang, Y. Liu, et al., Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2020.04.042; (e) J.Y. Chen, H.Y. Wu, Q.W. Gui, et al., Org. Lett. 22(2020) 2206-2209. |
| [12] |
X.Q. Mou, L. Xu, S.H. Wang, C. Yang, Tetrahedron Lett. 56 (2015) 2820-2822. DOI:10.1016/j.tetlet.2015.04.056 |
 2020, Vol. 31
2020, Vol. 31 


