b Jiangxi Key Laboratory for Mass Spectrometry and Instrumentation, East China University of Technology, Nanchang 330013, China;
c Green Catalysis Center, College of Chemistry, Zhengzhou University, Zhengzhou 450001, China
Acid catalysis is one of the most important and attractive fields of catalysis which has been widely applied in industry for the manufacture of various chemicals [1-3]. However, the traditional mineral acids (H2SO4, HF, etc.) and Lewis acids (AlCl3, BF3, etc.) are generally hazardous and corrosive which will lead to toxic and corrosive wastes [4]. Therefore, the development of green and environmentally friendly acidic catalytic systems including acidic ionic liquids and solid acids has drawn much attention in the past decades [5-14]. Among them, heteropoly acids (HPAs) and related polyoxometalate compounds (POMs) have attracted considerable interest from both academia and industry in the past decades due to their structural diversity and extensive applications in the fields of material chemistry [15-17], energy sciences [18, 19], CO2 chemistry [20-24], pharmaceutical chemistry [25] and catalytic chemistry [26-36]. Particularly, the employment of simple and easily available Keggin-type HPAs (e.g., H3PW12O40, H4SiW12O40 and H3PMo12O40) and their corresponding salts as catalysts has emerged as an efficient and versatile strategy for the synthesis of valuable organic compounds via C-C and C-N bonds formation [37-44].
Aldehydes are an important class of building blocks for the synthesis of fine chemicals and pharmaceuticals owing to their versatile reactivity. During the past centuries, a plethora of methods have been developed for the synthesis of aldehydes. For instance, the selective oxidation of the parent alkanes or alcohols [45-47], photocatalysis coupling of styrenes, and vinyl ethers [48], Pd/Rh-catalyzed reductive carbonylation of aryl halides [49]. Despite these significantachievements, the synthesisofpolysubstitutedaldehydes is stillvery challenging due to theirhigh reactivity [50, 51]. Therefore, the development of novel and efficient catalytic methods for the direct synthesis of polysubstituted aldehydes from commercially available starting materials is highly desired.
Inspired by the recent advances of polyoxometalate-catalyzed dehydrative coupling reactions of diarylmethanols [52-54], it is assumed that the soft polyanions might play unique roles in the generation of carbocation 6 from the corresponding secondary alcohol 1. On the other hand, in the presence of POMs catalyst the hydrolysis of epoxide 2 to give diol 4 should be possible [55, 56]. Subsequently, the diol 4 undergoes dehydration promoted by POMs to generate enol 5'. Then the coupling of the intermediate 5' and carbocation 6 gives the enol 7, which is further converted into the polysubstituted aldehydes 3 after tautomerization (Scheme 1a). However, there are no such reports on the synthesis of polysubstituted aldehydes from the corresponding secondary alcohols and epoxides/diols catalyzedbypolyoxometalates sofar.Herein, we report an efficient and general protocol catalyzed by the simple and inexpensive H3PMo12O40 for the synthesis of polysubstituted aldehydes through the reaction of diarylmethanols with epoxides 2, diols 4, or aldehydes 5 under mild reaction conditions (Scheme 1b).

|
Download:
|
| Scheme 1. Synthesis of polysubstituted aldehydes with polyoxometalate catalysis. | |
We set out to realize the dehydrative coupling reaction of diarylmethanol 1a and epoxide 2a to give α, β, β-polysubstituted aldehyde 3a under polyoxometalate catalysis. As shown in Table 1, the model reaction of 1a and 2a catalyzed by 3 mol% of HPAs (i.e., H3PW12O40, H4SiW12O40 and H3PMo12O40) in DCE at 80 ℃ for 2 h gave the desired product 3a in 54%, 66%, and 72% yields, respectively (entries 1-3). The control experiment without any catalyst did not provide the desired product 3a, indicating the significance of the polyoxometalate catalysis (entry 4). Further optimization of various solvents including H2O, CH3CN, PhCl, 1, 4- dioxane, THF, toluene, and CH3NO2 revealed that the polar CH3NO2 was the best solvent for this coupling reaction (entries 5-11). After systematic investigation, we were delighted to isolate the desired product 3a in 97% yield, when using 3 mol% of H3PMo12O40 as a catalyst, CH3NO2 as solvent at 90 ℃ for 2 h (entry 12). For more details, see Supporting information.
|
|
Table 1 Optimization of reaction conditions.a |
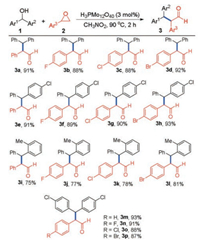
With the establishment of optimal reaction conditions, the substrate scope of this coupling reaction was then explored. As shown in Scheme 2, 91% isolated yield of the target product 3a was obtained from the model reaction. The epoxides bearing -F, -Cl, -Br groups on the para-position of benzene ring were converted into the corresponding polysubstituted aldehydes 3b-d in excellent yields (88%-92%). When (4-chlorophenyl)(phenyl)methanol was applied to react with various epoxides, the desired products 3e-h were obtained in 89%-93% yields. Phenyl(o-tolyl)methanol was also found to be suitable in this protocol to react with various epoxides delivering the corresponding aldehyde products 3i-l in moderate to good yields (75%-81%). Furthermore, the reactions of bis(4-chlorophenyl)methanol and various epoxides also worked smoothly affording the product 3m-p in good yields (87%-93%). Unfortunately, the aryl alcohols such as benzylic alcohol and 1-phenylethanoldid not react with epoxide 2a, suggesting that this protocol was limited in the diarylmethanols. In addition, when isopropanol and cyclohexanol were applied as the alcohol substrates under the standard conditions, no reaction was observed. These results suggest that the current catalytic system is not suitable for the aliphatic alcohols probably due to the inferior stability of the carbocation intermediate under the reaction conditions.
|
Download:
|
| Scheme 2. Substrate scope for the reaction of diarylmethanols and epoxides. Reaction conditions: 1 (0.2 mmol), 2 (0.24 mmol), H3PMo12O40 (3 mol%), CH3NO2 (1 mL), 90 ℃, 2 h. Isolated yields were given. | |
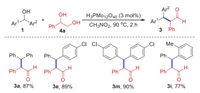
According to the proposed reaction pathway in Scheme 1, the diol 4 should be one of the possible intermediates. Therefore, 1-phenylethane-1, 2-diol 4a was employed as a substrate to react with various diarylmethanols 1 catalyzed by H3PMo12O40 under the standard conditions. As shown in Scheme 3, the polysubstituted aldehydes 3a, 3e, 3m, and 3i were obtained in good yields (77%-90%). These results suggested that the coupling of diarylmethanols and diols is an efficient strategy for the construction of polysubstituted aldehydes.
|
Download:
|
| Scheme 3. Substrate scope for the reaction of diarylmethanols and 1, 2-diol. Reaction conditions: 1 (0.2 mmol), 4 (0.24 mmol), H3PMo12O40 (3 mol%), CH3NO2 (1 mL), 90 ℃, 2 h. Isolated yields were given. | |
Additionally, the tautomerization of enol 5' produces the corresponding aldehyde 5, suggesting that the coupling of 2-arylacetaldehyde and diarylmethanols to synthesize the polysubstituted aldehydes should be possible in the presence of POM catalyst. Accordingly, when 2-phenylacetaldehyde 5a was applied as a substrate to react with diarylmethanols under standard conditions, the corresponding products 3n, 3o, 3p, and 3m were obtained in 87%-91% yields (Scheme 4). Compared with the previous report [50], this procedure provides a rapid and efficient method for the preparation of polyaryl-substituted aldehydes.

|
Download:
|
| Scheme 4. Substrate scope for the reaction of diarylmethanols and 2-phenylacetaldehyde. Reaction conditions: 1 (0.2 mmol), 5a (0.24 mmol), H3PMo12O40 (3 mol%), CH3NO2 (1 mL), 90 ℃, 2 h. Isolated yields were given. | |
Moreover, the gram-scale synthesis (5 mmol-scale) of the model product 3a from diarylmethanol 1a and the corresponding epoxide 2a or diol 4a was investigated under the standard conditions (Scheme 5). To our delight, this procedure was found to be practical and scalable as the desired product 3a were obtained in 87% and 82% yields, respectively.

|
Download:
|
| Scheme 5. Gram-scale synthesis. | |
In order to get a deeper insight into the mechanism for the above-mentioned dehydrative reaction, some control experiments were conducted as shown in Scheme 6. When the alcohol 1a was employed as the sole substrate under standard conditions, the corresponding ether 8 was isolated in 81% yield (Scheme 6a). Subsequently, when ether 8 was applied as a substrate to react with epoxide 2a and diol 4 under the standard conditions, the desired aldehyde products were isolated in yields of 90% and 89%, respectively (Scheme 6b and c). Such results indicated that ether 8 should be one of the reaction intermediates for the generation of carbocation 6, which is consistent with the previous results [52-54]. Moreover, the above-mentioned experimental results confirm that the proposed mechanism in Scheme 1a is reasonable.

|
Download:
|
| Scheme 6. Investigation of the reaction mechanism. | |
In conclusion, the coupling of diarylmethanols with epoxides (or diols, aldehydes) catalyzed by 3 mol% of heteropoly acid, i.e. H3PMo12O40, was established for the synthesis of aromatic polysubstituted aldehydes. Our route employs earth-abundant, readily accessible, and nontoxic heteropoly acid as catalyst, and it can be operated under mild reaction conditions providing various polysubstituted aldehydes in good to excellent yields. Importantly, this reaction could be scaled up to gram-scale. Such findings provide a new and green approach for the construction of aromatic polysubstituted aldehydes.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank the financial support from the National Natural Science Foundation of China (Nos. 21871026, 21971224), Research Found of East China University of Technology (Nos. DHBK2019265, DHBK2019267, DHBK2019264).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.018.
| [1] |
Y. Izumi, Catal. Today 33 (1997) 371-409. DOI:10.1016/S0920-5861(96)00165-4 |
| [2] |
P. Gupta, S. Paul, Catal. Today 236 (2014) 153-170. DOI:10.1016/j.cattod.2014.04.010 |
| [3] |
T. Akiyama, K. Mori, Chem. Rev. 115 (2015) 9277-9306. DOI:10.1021/acs.chemrev.5b00041 |
| [4] |
Y. Gu, F. Wu, J. Yang, Adv. Synth. Catal. 360 (2018) 2727-2741. DOI:10.1002/adsc.201800462 |
| [5] |
F. Su, Y. Guo, Green Chem. 16 (2014) 2934-2957. DOI:10.1039/C3GC42333F |
| [6] |
H. Marie, G. Alina, L. Brice, et al., ChemSusChem 11 (2018) 1249-1277. DOI:10.1002/cssc.201702435 |
| [7] |
A. Corma, H. García, Chem. Rev. 103 (2003) 4307-4365. DOI:10.1021/cr030680z |
| [8] |
M. Li, F. Wu, Y. Gu, Chin. J. Catal. 40 (2019) 1135-1140. DOI:10.1016/S1872-2067(19)63370-X |
| [9] |
A. Taheri, X. Pan, C. Liu, Y. Gu, ChemSusChem 7 (2014) 2094-2098. DOI:10.1002/cssc.201402220 |
| [10] |
C. Miao, H. Zhuang, Y. Wen, et al., Eur. J. Org. Chem. 2019 (2019) 3012-3021. DOI:10.1002/ejoc.201900169 |
| [11] |
C. Miao, Q. Hou, Y. Wen, et al., ACS Sustain. Chem. Eng. 7 (2019) 12008-12013. |
| [12] |
E.H.A. Yiliqi, B. Lai, L. Vaccaro, M. Li, Y. Gu, Adv. Synth. Catal. 361 (2019) 3342-3350. DOI:10.1002/adsc.201900246 |
| [13] |
Y. Sun, Q. Zhang, C. Zhang, et al., ACS Sustain. Chem. Eng. 7 (2019) 15114-15126. DOI:10.1021/acssuschemeng.9b03848 |
| [14] |
Q. Zhang, C. Zhang, Y. Sun, Y. Guo, D. Song, Appl. Catal. A Gen. 574 (2019) 10-24. DOI:10.1016/j.apcata.2019.01.021 |
| [15] |
D.L. Long, R. Tsunashima, L. Cronin, Angew. Chem. Int. Ed. 49 (2010) 1736-1758. DOI:10.1002/anie.200902483 |
| [16] |
P. Ma, F. Hu, J. Wang, J. Niu, Coord. Chem. Rev. 378 (2019) 281-309. DOI:10.1016/j.ccr.2018.02.010 |
| [17] |
Y. Chen, S. Sun, D. Lu, Y. Shi, Y. Yao, Chin. Chem. Lett. 30 (2019) 37-43. DOI:10.1016/j.cclet.2018.10.022 |
| [18] |
L. Chen, W.L. Chen, X.L. Wang, et al., Chem. Soc. Rev. 48 (2019) 260-284. DOI:10.1039/C8CS00559A |
| [19] |
Y. Ji, J. Hu, J. Biskupek, et al., Chem. Eur. J. 23 (2017) 16637-16643. DOI:10.1002/chem.201703851 |
| [20] |
Y. Cao, Q. Chen, C. Shen, L. He, Molecules 24 (2019) 2069. DOI:10.3390/molecules24112069 |
| [21] |
L.M. Sanchez, H.J. Thomas, M.J. Climent, G.P. Romanelli, S. Iborra, Catal. Rev. 58 (2016) 497-586. DOI:10.1080/01614940.2016.1248721 |
| [22] |
B. Yu, B. Zou, C.W. Hu, J. CO2 Util. 26 (2018) 314-322. DOI:10.1016/j.jcou.2018.05.021 |
| [23] |
Q. Huang, J. Liu, L. Feng, et al., Natl. Sci. Rev. 7 (2020) 53-63. |
| [24] |
L. Qiao, M. Song, A. Geng, S. Yao, Chin. Chem. Lett. 30 (2019) 1273-1276. DOI:10.1016/j.cclet.2019.01.024 |
| [25] |
I.A. Weinstock, R.E. Schreiber, R. Neumann, Chem. Rev. 118 (2018) 2680-2717. DOI:10.1021/acs.chemrev.7b00444 |
| [26] |
Y. Zhang, X. Chen, L. Li, et al., ACS Sustain. Chem. Eng. 7 (2019) 4975-4982. DOI:10.1021/acssuschemeng.8b05627 |
| [27] |
S.S. Wang, G.Y. Yang, Chem. Rev. 115 (2015) 4893-4962. DOI:10.1021/cr500390v |
| [28] |
G.P. Yang, S.X. Shang, B. Yu, C.W. Hu, Inorg. Chem. Front. 5 (2018) 2472-2477. DOI:10.1039/C8QI00678D |
| [29] |
Q.W. Song, B. Yu, X.D. Li, et al., Green Chem. 16 (2014) 1633-1638. DOI:10.1039/c3gc42406e |
| [30] |
C.X. Guo, B. Yu, J.N. Xie, L.N. He, Green Chem. 17 (2015) 474-479. DOI:10.1039/C4GC01638F |
| [31] |
H. Yu, J. Wang, Z. Wu, et al., Green Chem. 21 (2019) 4550-4554. DOI:10.1039/C9GC02053E |
| [32] |
Z. Wei, S. Ru, Q. Zhao, et al., Green Chem. 21 (2019) 4069-4075. DOI:10.1039/C9GC01248F |
| [33] |
K. Suzuki, N. Mizuno, K. Yamaguchi, ACS Catal. 8 (2018) 10809-10825. DOI:10.1021/acscatal.8b03498 |
| [34] |
A. Enferadi-Kerenkan, T.O. Do, S. Kaliaguine, Catal. Sci. Technol. 8 (2018) 2257-2284. DOI:10.1039/C8CY00281A |
| [35] |
K. Kamata, Bull. Chem. Soc. Jpn. 88 (2015) 1017-1028. DOI:10.1246/bcsj.20150154 |
| [36] |
Y.W. Peng, C. Shan, H.J. Wang, et al., Adv. Energy Mater. 9 (2019) 1900597. |
| [37] |
M. Egi, M. Umemura, T. Kawai, S. Akai, Angew. Chem. Int. Ed. 50 (2011) 12197-12200. DOI:10.1002/anie.201106381 |
| [38] |
J.S. Yadav, B.V.S. Reddy, P. Sridhar, et al., Eur. J. Org. Chem. (2004) 552-557. |
| [39] |
G.W. Wang, Y.B. Shen, X.L. Wu, Eur. J. Org. Chem. (2008) 4999-5004. |
| [40] |
G.W. Wang, Y.B. Shen, X.L. Wu, Eur. J. Org. Chem. (2008) 4367-4371. |
| [41] |
Y. Jia, Y. Fang, Y. Zhang, H.N. Miras, Y.F. Song, Chem. Eur. J. 21 (2015) 14862-14870. DOI:10.1002/chem.201501953 |
| [42] |
G.P. Yang, D. Dilixiati, T. Yang, et al., Appl. Organomet. Chem. 32 (2018) e4450. DOI:10.1002/aoc.4450 |
| [43] |
G.P. Yang, X. He, B. Yu, C.W. Hu, Appl. Organomet. Chem. 32 (2018) e4532. DOI:10.1002/aoc.4532 |
| [44] |
X. Feng, T. Yang, X. He, B. Yu, C.W. Hu, Appl. Organomet. Chem. 32 (2018) e4314. DOI:10.1002/aoc.4314 |
| [45] |
E. Gaster, S. Kozuch, D. Pappo, Angew. Chem. Int. Ed. 56 (2017) 5912-5915. DOI:10.1002/anie.201702511 |
| [46] |
K.J. Liu, S. Jiang, L.H. Lu, et al., Green Chem. 20 (2018) 3038-3043. DOI:10.1039/C8GC00223A |
| [47] |
X.J. Yang, Y.W. Zheng, L.Q. Zheng, et al., Green Chem. 21 (2019) 1401-1405. DOI:10.1039/C8GC03828G |
| [48] |
F. Wu, L. Wang, J. Chen, D.A. Nicewicz, Y. Huang, Angew. Chem. Int. Ed. 57 (2018) 2174-2178. DOI:10.1002/anie.201712384 |
| [49] |
M.Y.S. Ibrahim, S.E. Denmark, Angew. Chem. Int. Ed. 57 (2018) 10362-10367. DOI:10.1002/anie.201806148 |
| [50] |
C. Xing, H. Sun, J. Zhang, G. Li, Y.R. Chi, Chem. Eur. J. 17 (2011) 12272-12275. DOI:10.1002/chem.201102623 |
| [51] |
Q.Y. Lv, B. Yu, J. Liaocheng Univ. (Nat. Sci. Ed.) 32 (2019) 28-34. |
| [52] |
G.P. Yang, N. Zhang, N.N. Ma, B. Yu, C.W. Hu, Adv. Synth. Catal. 359 (2017) 926-932. DOI:10.1002/adsc.201601231 |
| [53] |
G.P. Yang, N. Jiang, X.Q. Huang, B. Yu, C.W. Hu, Mol. Catal. 468 (2019) 80-85. DOI:10.1016/j.mcat.2019.02.019 |
| [54] |
G.P. Yang, X. Wu, B. Yu, C.W. Hu, ACS Sustain. Chem. Eng. 7 (2019) 3727-3732. DOI:10.1021/acssuschemeng.8b06445 |
| [55] |
V.V. Costa, K.A. da Silva Rocha, I.V. Kozhevnikov, E.V. Gusevskaya, Appl. Catal. A:Gen. 383 (2010) 217-220. DOI:10.1016/j.apcata.2010.06.005 |
| [56] |
M.W.C. Robinson, A.M. Davies, R. Buckle, et al., Org. Biomol. Chem. 7 (2009) 2559-2564. DOI:10.1039/b900719a |
 2020, Vol. 31
2020, Vol. 31 


