b Center for Supramolecular Chemistry and Catalysis and Department of Chemistry, Shanghai University, Shanghai 200444, China;
c State Key Laboratory of NBC Protection for Civilian, Beijing 102205, China
As a new class of supramolecular macrocyclic hosts, pillararenes possess prism-like geometries and have π-rich cavities with multiple alkoxy or hydroxyl groups on both portals [1-11]. Pillararenes have shown excellent cavity host-guest properties towards a variety of guest molecules [12-22]. Compared with other popular macrocyclic receptors, the most peculiar binding behavior of pillararenes is the highly strong complexation of pillar[5]arenes (P5As) towards suitable neutral molecules in organic solution [23, 24]. Our group has developed a series of α, ω-disubstituted alkane guests for P5As [25-30]. The guests with four-methylene bridges, which length fits the height of P5As's cavity, give the strongest binding affinities.
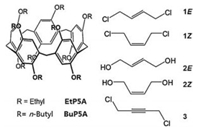
Based on these results, we wonder if carbon-carbon double and triple bonds can be engulfed by the cavities of P5As. In this work, we wish to report the host-guest properties of P5As (EtP5A and BuP5A) towards a series of olefin guests (Scheme 1), i.e., (E)-1, 4-dichlorobut-2-ene (1E), (Z)-1, 4-dichlorobut-2-ene (1Z), (E)-but-2-ene-1, 4-diol (2E), and (Z)-but-2-ene-1, 4-diol (2Z), as well as an alkyne 1, 4-dichlorobut-2-yne (3), exhibiting interesting binding selectivities of cis/trans olefins. Olefins are basic feedstocks in the chemical industry. The selective recognition of an isomer of an olefin over the other is a challenging task due to their similar chemical and physical properties. Very recently, our group [31] and Huang's group [32] demonstrated the separation of cis- and trans-olefin isomers by crystalline materials of biphen[3]arene [33-35] and P5As at the solid-vapor phase. To the best of our knowledge, the present work is the first example of selective binding of olefin isomers by pillararenes in solution.
|
Download:
|
| Scheme 1. Structures of the host and the guests. | |
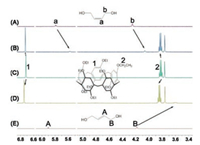
Fig. 1 shows the 1H NMR spectra of hydrocarbons 2Z and 2E in CDCl3 in the absence and presence of 1.0 equiv. amount of EtP5A host. Compared to the free 2Z and 2E (Figs. 1A and E), the peaks for the protons of 2Z and 2E (Figs. 1B and D) exhibit upfield shifts and broadening as a consequence of inclusion-induced shielding effects. Meanwhile, the host is deshielded by the presence of the guests, since proton signals of EtP5A derived from aromatic protons shift downfield. Therefore, we can deduce that the guest molecules are included in the cavity of the host. In addition, the chemical shifts induced by the cis- and trans-guest are different. The encapsulation-induced upfield shifts and broadening effects of methylene protons of 2E are more obvious than those of 2Z. For example, the ∆δ value for HB of 2E (-0.59 ppm) is much larger than that for Hb of 2Z (-0.18 ppm). And the middle alkenyl protons (HA) of 2E exhibit the most remarkable complexation-induced peak broadening, because their signals cannot be observed in the 1H NMR spectrum. Correspondingly, the downfield shifts of aromatic and methylene protons (H1, H2) of EtP5A caused by the trans- guest 2E (∆δH1 = 0.027 ppm, ∆δH2 = 0.020 ppm) are more obvious than those caused by the cis-guest 2Z (∆δH1 = 0.008 ppm, ∆δH2 = 0.006 ppm). These results suggest that the complexation between the host and the trans-olefin guest 2E is stronger than that for the cis-2Z. The interactions of EtP5A with the other olefin guests (1E, 1Z), as well as BuP5A with 1E, 1Z, 2E and 2Z were also investigated. Similar NMR changes were observed, wherethe encapsulation-induced NMR changes of trans-guests were always lager that for cis- ones.
|
Download:
|
| Fig. 1. 1H NMR spectra (500 MHz, 298 K) of (A) 2Z, (B) 2Z+ EtP5A, (C) EtP5A, (D) 2E+ EtP5A, (E) 2E in CDCl3 at 5.0 mmol/L. | |
1H NMR results suggested that both cis- and trans-olefins can be engulfed in P5As, however, their binding affinities were quite different. The association constants for the four olefin guests with two P5As in CDCl3 were then tested by NMR titration methods (Table 1). The values, ranging from 10 L/mol to 210 L/mol, are lower than that for alkane compounds such as 1, 4-dichlorobutane and 1, 4-butanediol (Table S1 in Supporting information). This may be due to the fact that the milldle C-H bonds in olefins are less than those in the alkane guests, resulting in a smaller number of hostguest C-H… π and C-H…O hydrogen bonds. Another possible reason is that, in comparison with olefins, alkane derivatives could effectively adjust themselves to fit the host cavity well due to their more flexible backbones.
|
|
Table 1 Association constants (Ka) for the host-guest inclusion complexation in CDCl3 at 298 K. |
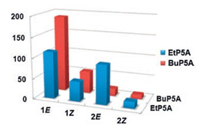
For both P5As hosts, the association constants of trans-isomers are larger than those of their cis-isomers (Fig. 2). For example, when BuP5A gives a 1E/1Z selectivity of 3.8, the trans-/cis-isomer selectivity of 2E/2Z by EtP5A is up to 6.3. This is reasonable that trans-isomers having stretching structures are more suitable for P5As's cavity than cis-isomers. The non-centrosymmetric structure of cis-guest leads to a lower host-guest induced-fit degree and has higher steric hindrance during the complexation, ultimately leading to a weaker host-guest interaction.
|
Download:
|
| Fig. 2. Bar graph of the Ka values of the hosts EtP5A (blue bar) and BuP5A (red bar) and four olefinic guests 1E, 1Z, 2E and 2Z. | |
The size/shape fit between trans-isomer and P5A cavity was then confirmed by a single crystal structure of 1E⊂BuP5A. Guests 1E and BuP5A (1:1, molar ratio) were completely dissolved in methylene chloride, and then covered with a layer of ethyl acetate solution at room temperature. We successfully obtained single crystals of 1E⊂BuP5A complex suitable for X-ray analysis. It was found that there are nine (week) C-H…Cl hydrogen bonding interactions between the two chlorine atoms of 1E and BuP5A's two portals (Fig. 3). Besides, there exist quadruple C-H…O and quadruple C-H…π interactions between the guest protons with the host's oxygen atoms and benzenes. The main driving forces of host-guest encapsulation are multiple C-H…Cl (O) and C-H…π interactions.

|
Download:
|
| Fig. 3. Single-crystal structure of 1E⊂BuP5A. Pink dashes (A-I) represent C-H…Cl, bright blue dashes (a-d) represent C-H…π and green dashes (e-h) represent C-H…O interactions between 1E and BuP5A. | |
Crystallographic data for 1E ⊂BuP5A CCDC: 1962361: colorless, C79H116Cl2O10, FW 1296.61, triclinic, space group P-1, a = 12.214(2), b = 14.805(3), c = 21.747(4), α = 89.735(3), β = 89.579(3), γ = 75.559 (3), v = 3808.3(11) Å3, Z = 2, Dc =1.131 g/cm3, T = 173(2) K, μ = 0.140 mm-1, 22, 779 measured reflections, 13, 315 independent reflections, 846 parameters, 9 restraint, F000 = 1408, R1 = 0.1453, wR2 = 0. 0.3355 (all data), R1 = 0.1023, wR2 = 0.2829 [I> 2σ(I)], max. residual density 1.084 e/Å3, and goodness-of-fit (F2) = 1.031.
After studying these olefin guests, we also examined if carboncarbon triple bond can be included in P5As cavity. An alkyne compound, 1, 4-dichloro-2-butyne (3) was chosen as the guest. The complexation-induced chemical shifts of EtP5A and BuP5A with 3 in the NMR spectrum were so small that the Ka values for 3 are very small and cannot be determined accurately. No C-H…π interactions can be formed between the middle sp bonding carbon atoms of 3 and benzene rings of P5As.
In summary, we report here that besides well reported α, ω-disubstituted alkane guests, olefin guests bearing doubledouble bonds can also be efficiently bound by the cavities of P5As to form inclusion complexes in organic solution. Host-guest C-H… π and C-H…O hydrogen bonding interactions provide the main driving forces for the binding events. Particularly, the association constants of trans-isomers are higher than those of cis-ones for all the host-guest pairs, and the largest isomer selectivity of 2E/2Z by EtP5A is up to 6.3. This work expands the guest moieties for pillar[5]arenes from alkane derivatives to olefins. The interesting binding selectivity of cis-/trans-isomers could find potential applications in olefin separation.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThe authors gratefully acknowledge the National Natural Science Foundation of China (Nos. 21772118 and 21971192).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.02.037.
| [1] |
T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc. 130 (2008) 5022-5023. DOI:10.1021/ja711260m |
| [2] |
M. Zhang, P.P. Zhu, P. Xin, et al., Angew. Chemie. 129 (2017) 3045-3049. DOI:10.1002/ange.201612093 |
| [3] |
K. Yang, Y. Pei, J. Wen, Z. Pei, Chem. Commun. (2016) 9316-9326. |
| [4] |
M. Ni, N. Zhang, W. Xia, et al., J. Am. Chem. Soc. 138 (2016) 6643-6649. DOI:10.1021/jacs.6b03296 |
| [5] |
N. Song, T. Kakuta, T. Yamagishi, Y.W. Yang, T. Ogoshi, Chem 4 (2018) 2029-2053. DOI:10.1016/j.chempr.2018.05.015 |
| [6] |
H. Zhang, Z. Liu, Y. Zhao, Chem. Soc. Rev. 47 (2018) 5491-5528. DOI:10.1039/C8CS00037A |
| [7] |
T. Xiao, L. Zhou, X.Q. Sun, et al., Chin. Chem. Lett. 31 (2020) 1-9. DOI:10.1016/j.cclet.2019.05.011 |
| [8] |
Y. Han, C.Y. Nie, S. Jiang, J. Sun, C.G. Yan, Chin. Chem. Lett. 31 (2020) 725-728. DOI:10.1016/j.cclet.2019.09.014 |
| [9] |
Z. Liu, J. Wu, C. Wang, et al., Chin. Chem. Lett. 30 (2019) 2299-2303. DOI:10.1016/j.cclet.2019.10.023 |
| [10] |
K. Jie, Y. Zhou, E. Li, F. Huang, Acc. Chem. Res. 51 (2018) 2064-2072. DOI:10.1021/acs.accounts.8b00255 |
| [11] |
T. Xiao, L. Qi, W. Zhong, et al., Mater. Chem. Front. 3 (2019) 1973-1993. DOI:10.1039/C9QM00428A |
| [12] |
T. Xiao, W. Zhong, L. Xu, et al., Org. Biomol. Chem. 17 (2019) 1336-1350. DOI:10.1039/C8OB03095B |
| [13] |
T. Xiao, L. Zhou, L. Xu, et al., Chin. Chem. Lett. 30 (2019) 271-276. DOI:10.1016/j.cclet.2018.05.039 |
| [14] |
C. Ke, N.L. Strutt, H. Li, et al., J. Am. Chem. Soc. 135 (2013) 17019-17030. DOI:10.1021/ja407229h |
| [15] |
Z. Zhang, Y. Luo, J. Chen, et al., Angew. Chem. Int. Ed. 50 (2011) 1397-1401. DOI:10.1002/anie.201006693 |
| [16] |
T. Ogoshi, T. Yamagishi, Y. Nakamoto, Chem. Rev. 116 (2016) 7937-8002. DOI:10.1021/acs.chemrev.5b00765 |
| [17] |
B. Li, Z. Meng, Q. Li, et al., Chem. Sci. 8 (2017) 4458-4464. DOI:10.1039/C7SC01438D |
| [18] |
K. Jie, Y. Zhou, E. Li, et al., J. Am. Chem. Soc. 140 (2018) 3190-3193. DOI:10.1021/jacs.7b13156 |
| [19] |
J. Chen, H. Ni, Z. Meng, et al., Nat. Commun. 10 (2019) 3546. DOI:10.1038/s41467-019-11553-7 |
| [20] |
X.Q. Wang, W. Wang, W.J. Li, et al., Nat. Commun. 9 (2018) 3190. DOI:10.1038/s41467-018-05670-y |
| [21] |
D. Cao, Y. Kou, J. Liang, et al., Angew. Chem. Int. Ed. 48 (2009) 9721-9723. DOI:10.1002/anie.200904765 |
| [22] |
Q. Hao, Y. Chen, Z. Huang, et al., ACS Appl. Mater. Interfaces 10 (2018) 5365-5372. DOI:10.1021/acsami.7b19784 |
| [23] |
X. Shu, J. Fan, J. Li, et al., Org. Biomol. Chem. 10 (2012) 3393-3397. DOI:10.1039/c2ob25251a |
| [24] |
Y. Wang, G. Ping, C. Li, Chem. Commun. 52 (2016) 9858-9872. DOI:10.1039/C6CC03999E |
| [25] |
C. Li, L. Zhao, J. Li, et al., Chem. Commun. 46 (2010) 9016-9018. DOI:10.1039/c0cc03575k |
| [26] |
C. Li, S. Chen, J. Li, et al., Chem. Commun. 47 (2011) 11294-11296. DOI:10.1039/c1cc14829j |
| [27] |
X. Shu, S. Chen, J. Li, et al., Chem. Commun. 48 (2012) 2967-2969. DOI:10.1039/c2cc00153e |
| [28] |
C. Li, K. Han, J. Li, et al., Org. Lett. 14 (2012) 42-45. DOI:10.1021/ol2027834 |
| [29] |
C. Li, K. Han, J. Li, et al., Chem. Eur. J. 19 (2013) 11892-11897. DOI:10.1002/chem.201301022 |
| [30] |
X. Wang, K. Han, J. Li, X. Jia, C. Li, Polym. Chem. 4 (2013) 3998-4003. DOI:10.1039/c3py00462g |
| [31] |
Y. Wang, K. Xu, B. Li, et al., Angew. Chem. Int. Ed. 58 (2019) 10281-10284. DOI:10.1002/anie.201905563 |
| [32] |
Y. Zhou, K. Jie, R. Zhao, F. Huang, J. Am. Chem. Soc. 141 (2019) 11847-11851. DOI:10.1021/jacs.9b06188 |
| [33] |
H. Chen, J. Fan, X. Hu, et al., Chem. Sci. 6 (2015) 197-202. DOI:10.1039/C4SC02422B |
| [34] |
B. Li, B. Wang, X. Huang, et al., Angew. Chem. Int. Ed. 58 (2019) 3885-3889. DOI:10.1002/anie.201813972 |
| [35] |
K. Xu, Z.Y. Zhang, C. Yu, et al., Angew. Chem. Int. Ed. 59 (2020) 7214-7218. DOI:10.1002/anie.202000909 |
 2020, Vol. 31
2020, Vol. 31 


