b Department of Chemistry, College of Science, China University of Petroleum (East China), Qingdao 266580, China
Supramolecular systems with dynamical and reversible properties have been widely applied to prepare advanced functional materials [1-7]. Macrocycles are important building blocks for the fabrication of supramolecular materials with desired properties and promising functions [8, 9]. Stimuli-responsiveness originated from macrocycle-based molecular recognition motifs is an essential part of supramolecular systems [10-14].
Incorporating different types of macrocyclic structures into a single molecule is an effective strategy to combine different properties and functions together [15-17]. The coexistence of various macrocycles not only introduces diverse stimuli-responsiveness, but provides extra supramolecular control over the selfassembly [18-20]. Especially, multiple stimuli-responsiveness can be realized by the coexistence of different macrocycles via the orthogonal molecular recognition [21-23]. The introduction of multiple macrocycles into single molecules dramatically expands the selectivity and cooperation of host–guest recognition.
Crown ethers [24-27], cyclodextrins [28, 29], calix[n]arenes [30, 31], pillar[n]arenes [21, 23, 25, 29], or cucurbit[n]urils [32] are widely selected to prepare stimuli-responsive materials. However, among commonly used macrocycles, pillar[n]arenes are less explored to realize multiple responsiveness along with different macrocycles. Only a limited number of examples were reported [25, 27, 29, 33-35]. Previously, Wen and co-workers synthesized pillar[5]arene-crown ether fused macrocycles [27, 33-35]. Chen et al. reported artificial channels by pillararene-cyclodextrin molecules [29]. Considering that more macrocycles give rise to more flexibility for realizing stimuli-responsiveness and supramolecular control, it is of great importance to design pillar[n]arenebased stimuli-responsive systems with different macrocycles [36].
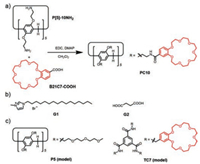
We here report a crown ether-pillararene hybrid system with LCST-type thermo-responsiveness. A pillar[5]arene equipped with ten benzo-21-crown-7 (B21C7) units PC10 was designed and prepared by the simple amidation reaction as depicted in Scheme 1 with a yield of 45%. Thermo-responsiveness of PC10 is not only controlled by the B21C7-involved host–guest interactions, but further manipulated by the pillar[5]arene-based supramolecular complexation.
|
Download:
|
| Scheme 1. (a) Synthetic route of PC10; chemical structures of (b) G1 and G2; (c) P5 and TC7. | |
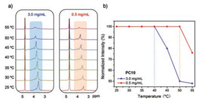
At room temperature, PC10 exhibits a good solubility (125 mg/mL) in water, which was confirmed by 1H NMR and macroscopic test (Fig. 1 and Fig. S2 in Supporting information). Intriguing, the clear solution turns to opaque upon heating and returns back to transparent after cooling, displaying a typical LCST behavior (Fig. 1a) [37-39]. The transmittance-temperature curves were determined by UV–vis measurement and show that the higher the concentration of PC10 is, the lower Tcloud value becomes (Fig. 1b and Fig. S4 in Supporting information). For example, Tcloud of PC10 at 3.0 mg/mL (0.63 mmol/L) is 46.2 ℃ with a sharp transition (< 1.0 ℃). It should be noted that PC10 can display the macroscopic phase transition at an extremely low concentration (64.9 ℃, 0.1 mg/mL, Fig. S4). Furthermore, a narrow hysteresis (< 2.0 ℃) appears during the heating and cooling cycle (Fig. 1c). Different heating rates show negligible influences on Tcloud (Fig. 1d). The dynamic light scattering (DLS) of PC10 was carried out and the results are in good agreement with the UV–vis measurements (Fig. S5 in Supporting information).

|
Download:
|
| Fig. 1. (a) The macroscopic photos of LCST behavior; (b) concentration-dependent LCST behavior of PC10; (c) turbid curves of PC10 during the heating and cooling cycle; (d) turbid curves of PC10 with different heating rates. | |
To provide an insight view of the phase transition behavior, attentions were then focused on the temperature-dependent 1H NMR measurements (Fig. 2a, Figs. S6 and S7 in Supporting information). The proton signal intensity of PC10 decays upon heating. The normalized intensities of the proton signals at different temperatures were plotted (Fig. 2b). Compared to the PC10 solution with a higher concentration (3.0 mg/mL), a delayed occurrence of decreased normalized intensity was observed in the PC10 solution with a lower concentration (0.5 mg/mL), which demonstrates that higher temperature is necessary to realize the phase separation of a lower concentration solution [40].
|
Download:
|
| Fig. 2. (a) Partial temperature-dependent 1H NMR spectra (400 MHz, D2O) of PC10; (b) normalized intensity of PC10 at different concentrations. | |
Previous studies have demonstrated that B21C7 can form complexes with K+ due to the suitable cavity size and geometry, while has no interaction with Na+ [41-44]. Sodium chloride and potassium chloride as the sources of K+ and Na+ were added to the solution of PC10 to investigate the ion-responsiveness, respectively. As shown in Fig. 3a, the addition of K+ or Na+ to the solution of PC10 (3.0 mg/mL, 0.63 mmol/L) leads to obviously different phenomena. After the addition of Na+, Tcloud was tuned from 46.2 ℃ to 21.7 ℃, displaying a typical salting-out effect. However, Tcloud of PC10 at the presence of K+ is more complicated. Tcloud initially increases and shows a salting-ineffect (stage1, < 1.5mol/L). When Tcloud reaches a maximum, the further addition of K+resultsin a decrease of Tcloud (salting-out effect, stage 2, > 1.5 mol/L) [42, 43]. The blue dots clearly show that two close Tcloud values were observed at two stages, when the K+ concentrations are quite different(Fig. 3a)[42, 43].From Figs. 3b and c(details in Figs.S8 and S9 in Supporting information), it is obvious that the normalized intensities of PC10 remain constant at low temperature, thenstartto decrease as temperature increased. An earlier appearance of the decreased normalized intensity of PC10 was observed at the presence of Na+ (68.5 mmol/L), compared with the pure PC10 solution. For the solution of PC10 with K+ (53.6 mmol/L), a delayed occurrence of the decreased intensity was observed. These observations are consistent with the UV–vis measurements.

|
Download:
|
| Fig. 3. (a) Tcloud of PC10 with different salts; (b) normalized intensity of PC10 with different salts. The concentration of PC10 is 0.63 mmol/L; (c) partial temperaturedependent 1H NMR spectra (400 MHz, D2O) of PC10, PC10@68.5 mmol/L NaCl and PC10@53.6 mmol/L KCl. The red x symbols in (a) mean that the Tcloud was higher than 85 ℃ and difficult to be recorded. | |
Na+ (no complexation with B21C7) interacts with the surrounding water molecules, effectively reduces the number of water molecules that forms hydrogen bonds with PC10, and eventually leads to the lower Tcloud (salting-out effect) [44]. K+ can form complexes with PC10 and the complexation percentages increase at stage 1. Therefore, the complexes of PC10@K+ are more difficult to aggregate together due to electrostatic repulsion, indicating that higher temperature is necessary to destroy complexes to generate enough free PC10 to realize the transparent-turbid transitions (salting-in effect) [44]. Since the number of the crown ethers is limited, once all crown ethers are occupied by K+, Tcloud reaches a maximum value. Further addition of K+ only leads to a lower Tcloud (salting-out effect).
The charge and the geometry of pillararene-based host–guest pair have exerted great influences on the LCST behavior [41-52]. In this study, water soluble ionic liquids imidazole salt (G1) and neutral succinic acid (G2) were used to investigate the LCST behavior in the presence/absence of supramolecular complexation. G1 has the host–guest interactions with PC10 in water due to the hydrophobic effect and the electrostatic interaction (Fig. S10 in Supporting information). No binding capacity was observed between PC10 and G2 (Fig. S11 in Supporting information). As expected, Tcloud increases significantly in response to the addition of G1 (Fig. 4a) [47, 48]. Based on the temperature-dependent NMR spectra, the normalized intensity of PC10 was plotted (Figs. S15 and S16 in Supporting information). The decreased intensity of PC10@G1 appears at higher temperature, compared with that of the pure PC10 solution. These observations are consistent with UV–vis measurements and can be ascribed to the electrostatic repulsion of G1, which makes it more difficult for PC10@G1 to aggregate together to realize LCST behavior [49]. This upward Tcloud curve is also consistent with our previous results in pillar[5]arene/ionic liquids solution [51]. However, Tcloud shows remarkable decreases when G2 was added to the solution of PC10 (Fig. 4b). Since no host–guest interactions were observed between PC10 and G2, the decreased Tcloud should be ascribed to other factors. Two control compounds, P5 (only has the pillar[5]arene unit) and TC7 (only has B21C7 units) were applied. Neither P5 nor TC7 shows increased Tcloud, even when excess G2 was added (Fig. 4b). These control experiments further confirm that no host–guest complexation occurs between pillararene and G2 or between B21C7 and G2.

|
Download:
|
| Fig. 4. Tcloud of (a) PC10 (0.63 mmol/L)@G1; (b) G2 with different hosts. Partial 1H NMR spectra (400 MHz, D2O) of the titration experiments of (c) PC10 (0.63 mmol/L) @G2; (d) P5 (0.63 mmol/L)@G2; (e) TC7 (2.1 mmol/L)@G2. | |
1H NMR titration measurements between G2 and different hosts were later applied (Figs. 4c–e, Figs. S12–S14 in Supporting information). In PC10@G2 solution, the proton signal intensity of crown ethers decays along with the increased G2 concentration (Fig. 4c). However, no changes of the normalized intensities of P5 or TC7 were observed, when an excess amount of G2 was added to P5 or TC7 solutions respectively (Figs. 4d and e). As shown in Fig. 5a, the normalized intensity keeps unchangeable for G2@P5 or G2@TC7 solutions, respectively. More information is obtained from temperature-dependent NMR measurements. In PC10@G2 solution, the normalized proton signal intensity of crown ethers decays along with the increased temperature (Figs. 5b and c, Figs. S17–S22 in Supporting information). All these observations confirm that the addition of G2 results in the aggregation of PC10 at both room temperature and high temperature. On the contrast, no aggregation of P5 or TC7 was observed, when G2 was added. This information demonstrates that the decreased Tcloud of PC10 is caused by the salting-out effect of G2. Unlike the LCST behavior of reported pillararenes with glycol chains (which are regulated by the host–guest interactions), the LCST behavior of PC10 can be affected even without the host–guest interactions [37, 51].

|
Download:
|
| Fig. 5. Normalized intensity of (a) G2 with different hosts; (b) PC10 (0.63 mmol/L) with different concentrations of G2; (c) partial temperature-dependent 1H NMR spectra (400 MHz, D2O) of PC10 (0.63 mmol/L) with different concentrations of G2. | |
By analyzing the chemical structures of PC10, P5, and TC7, the difference is that PC10 has both pillar[5]arene and B21C7 units, while P5 or TC7 only contains pillar[5]arene or B21C7 units, respectively. A reasonable explanation is that the combination of the pillar[5]arene and B21C7 units can amplify the salting-out effect of G2. In our previous work, we have found that once the B21C7 units were connected to traditional polymers (every polymer chain contains 10–40 B21C7 units), the polymer-B21C7 system was more sensitive to guests and effectively enhanced and amplified the thermo-responsiveness of B21C7 [42]. In PC10 system, ten B21C7 units were connected to a single core (the pillar[5]arene core). Such a monomer structure is similar to the reported B21C7-polymer system. Hence compared with P5 or TC7, PC10 is more sensitive to G2, which leads to a rapid decrease of Tcloud (salting-out effect).
In conclusion, a new and water-soluble pillar[5]arene with deca (B21C7) units was developed. The LCST behaviors of this macrocyclic molecule were reversibly controlled by changing concentrations or adding cations or guests. Importantly, Tcloud shows a response to the absence of the host–guest interactions (PC10@G2) and displays an opposite trend to the presence of the host–guest interactions (PC10@G1). Furthermore, no remarkable decreases of Tcloud were observed in two model compound solutions (P5 and TC7), which demonstrates that mono-type hosts exert weak influence on Tcloud in G2 solutions. This hybrid system, which contains ten B21C7 units, can amplify the salting-out effect. Considering the facile route to adjust LCST, we expect this hybrid system can be an ideal candidate for the construction of functional intelligent materials.
Declaration of competing interestThe authors declare that there are no conflicts of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21704024), the Huxiang Young Talent Program from Hunan Province (No. 2018RS3036), the Fundamental Research Funds for the Central Universities from Hunan University and National Natural Science Foundation of Shandong Province (No. ZR2019QB024).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.074.
| [1] |
M. Zhang, P.P. Zhu, P. Xin, et al., Angew. Chem. Int. Ed. 56 (2017) 2999-3003. DOI:10.1002/anie.201612093 |
| [2] |
T. Xiao, W. Zhong, L. Zhou, et al., Chin. Chem. Lett. 30 (2019) 31-36. DOI:10.1016/j.cclet.2018.05.034 |
| [3] |
C.C. Zhang, Y.M. Zhang, Y. Liu, Chem. Commun. 54 (2018) 13591-13594. DOI:10.1039/C8CC08260J |
| [4] |
M. Guo, X. Wang, C. Zhan, et al., J. Am. Chem. Soc. 140 (2018) 74-77. DOI:10.1021/jacs.7b10767 |
| [5] |
L. Chen, J. Xiang, Y. Zhao, et al., J. Am. Chem. Soc. 140 (2018) 7079-7082. DOI:10.1021/jacs.8b04569 |
| [6] |
L. Xu, Z. Wang, R. Wang, et al., Angew. Chem. Int. Ed. 59 (2020) 9908-9913. DOI:10.1002/anie.201907678 |
| [7] |
D. Dai, Z. Li, J. Yang, et al., J. Am. Chem. Soc. 141 (2019) 4756-4763. DOI:10.1021/jacs.9b01546 |
| [8] |
S. Dong, Y. Luo, X. Yan, et al., Angew. Chem. Int. Ed. 50 (2011) 1905-1909. DOI:10.1002/anie.201006999 |
| [9] |
X. Ji, M. Ahmed, L. Long, et al., Chem. Soc. Rev. 48 (2019) 2682-2697. DOI:10.1039/C8CS00955D |
| [10] |
Y. Chen, S. Sun, D. Lu, et al., Chin. Chem. Lett. 30 (2019) 37-43. DOI:10.1016/j.cclet.2018.10.022 |
| [11] |
C. Li, Chem. Commun. 50 (2014) 12420-12433. DOI:10.1039/C4CC03170A |
| [12] |
S. Sun, M. Geng, L. Huang, et al., Chem. Commun. 54 (2018) 13006-13009. DOI:10.1039/C8CC07658H |
| [13] |
J.S. Cui, Q.K. Ba, H. Ke, et al., Angew. Chem. Int. Ed. 57 (2018) 7809-7814. DOI:10.1002/anie.201803349 |
| [14] |
Y.M. Zhang, N.Y. Zhang, K. Xiao, et al., Angew. Chem. Int. Ed. 57 (2018) 8649-8653. DOI:10.1002/anie.201804620 |
| [15] |
S.K. Kim, J.L. Sessler, Chem. Soc. Rev. 39 (2010) 3784-3809. DOI:10.1039/c002694h |
| [16] |
W.B. Hu, W.J. Hu, Y.A. Liu, et al., Chem. Commun. 52 (2016) 12130-12142. DOI:10.1039/C6CC03651A |
| [17] |
C. Lu, M. Zhang, D. Tang, et al., J. Am. Chem. Soc. 140 (2018) 7674-7680. DOI:10.1021/jacs.8b03781 |
| [18] |
J.L. Sessler, S.K. Kim, D.E. Gross, et al., J. Am. Chem. Soc. 130 (2008) 13162-13166. DOI:10.1021/ja804976f |
| [19] |
S.-K. Kim, J.L. Sessler, Acc. Chem. Res. 47 (2014) 2525-2536. DOI:10.1021/ar500157a |
| [20] |
L. Xu, X. Shen, Z. Zhou, et al., J. Am. Chem. Soc. 140 (2018) 16920-16924. DOI:10.1021/jacs.8b10842 |
| [21] |
P. Li, Y. Chen, Y. Liu, Chin. Chem. Lett. 30 (2019) 1190-1197. DOI:10.1016/j.cclet.2019.03.035 |
| [22] |
I.W. Park, J. Yoo, B. Kim, et al., Chem. Eur. J. 18 (2012) 2514-2523. DOI:10.1002/chem.201103239 |
| [23] |
X. Yan, P. Wei, Z. Li, et al., Chem. Commun. 49 (2013) 2512-2514. DOI:10.1039/c3cc40474a |
| [24] |
C. Hocquelet, J. Blu, C.K. Jankowski, et al., Tetrahedron 62 (2006) 11963-11971. DOI:10.1016/j.tet.2006.09.089 |
| [25] |
W.X. Feng, Z. Sun, Y. Zhang, et al., Org. Lett. 19 (2017) 1438-1441. DOI:10.1021/acs.orglett.7b00352 |
| [26] |
X. He, W.H. Lam, N. Zhu, et al., Chem. Eur. J. 15 (2009) 8842-8851. DOI:10.1002/chem.200900422 |
| [27] |
W.B. Hu, W.J. Hu, Y.A. Liu, et al., Org. Lett. 17 (2015) 2940-2943. DOI:10.1021/acs.orglett.5b01209 |
| [28] |
L. Gallego-Yerga, M. Lomazzi, F. Sansone, et al., Chem. Commun. 50 (2014) 7440-7443. DOI:10.1039/C4CC02703E |
| [29] |
P. Xin, H. Kong, Y. Sun, et al., Angew. Chem. Int. Ed. 58 (2019) 2779-2784. DOI:10.1002/anie.201813797 |
| [30] |
N. Sieffert, G. Wipff, J. Phys. Chem. B 110 (2006) 19497-19506. DOI:10.1021/jp063045g |
| [31] |
M. Massaro, V. Cinà, M. Labbozzetta, et al., RSC Adv. 6 (2016) 50858-50866. DOI:10.1039/C6RA06143E |
| [32] |
Y.M. Zhang, Z. Wang, Y. Chen, et al., Org. Biomol. Chem. 12 (2014) 2559-2567. DOI:10.1039/c3ob42103a |
| [33] |
W.B. Hu, H.M. Yang, W.J. Hu, et al., Chem. Commun. 50 (2014) 10460-10463. DOI:10.1039/C4CC01810A |
| [34] |
W.B. Hu, W.J. Hu, X.L. Zhao, et al., Chem. Commun. 51 (2015) 13882-13885. DOI:10.1039/C5CC05623C |
| [35] |
W.B. Hu, C.D. Xie, W.J. Hu, et al., J. Org. Chem. 80 (2015) 7994-8000. DOI:10.1021/acs.joc.5b01038 |
| [36] |
L. Chen, Y. Cai, W. Feng, et al., Chem. Commun. 55 (2019) 7883-7898. DOI:10.1039/C9CC03292D |
| [37] |
X. Ji, J. Chen, X. Chi, et al., ACS Macro Lett. 3 (2014) 110-113. DOI:10.1021/mz400528a |
| [38] |
S. Wang, C. Yao, M. Ni, et al., Polym. Chem. 8 (2017) 682-688. DOI:10.1039/C6PY01961G |
| [39] |
B. Zheng, Z. Luo, Y. Deng, et al., Chem. Commun. 55 (2019) 782-785. DOI:10.1039/C8CC09160A |
| [40] |
Y. Deng, X. Li, Q. Zhang, et al., Beilstein J. Org. Chem. 15 (2019) 437-444. DOI:10.3762/bjoc.15.38 |
| [41] |
P. Wei, T.R. Cook, X. Yan, et al., J. Am. Chem. Soc. 136 (2014) 15497-15500. DOI:10.1021/ja5093503 |
| [42] |
D. Huang, Q. Zhang, Y. Deng, et al., Polym. Chem. 9 (2018) 2574-2579. DOI:10.1039/C8PY00412A |
| [43] |
Z. Qi, L. Chiappisi, H. Gong, et al., Chem. Eur. J. 24 (2018) 3854-3861. DOI:10.1002/chem.201705838 |
| [44] |
Z. Luo, Y. Deng, X. Li, et al., New J. Chem. 43 (2019) 6890-6896. DOI:10.1039/C9NJ00846B |
| [45] |
S. Dong, B. Zheng, Y. Yao, et al., Adv. Mater. 25 (2013) 6864-6867. DOI:10.1002/adma.201303652 |
| [46] |
X. Liao, L. Guo, J. Chang, et al., Macromol. Rapid Commun. 36 (2015) 1492-1497. DOI:10.1002/marc.201500167 |
| [47] |
X. Chi, X. Ji, D. Xia, et al., J. Am. Chem. Soc. 137 (2015) 1440-1443. DOI:10.1021/ja512978n |
| [48] |
S. Dong, J. Heyda, J. Yuan, et al., Chem. Commun. 52 (2016) 7970-7973. DOI:10.1039/C6CC02838A |
| [49] |
X. Chi, G. Yu, L. Shao, et al., J. Am. Chem. Soc. 138 (2016) 3168-3174. DOI:10.1021/jacs.5b13173 |
| [50] |
L.P. Yang, H. Liu, S.B. Lu, et al., Org. Lett. 19 (2017) 1212-1215. DOI:10.1021/acs.orglett.7b00181 |
| [51] |
L. Wang, X. Li, Q. Zhang, et al., New J. Chem. 42 (2018) 8330-8333. DOI:10.1039/C8NJ01366G |
| [52] |
T. Xiao, L. Zhou, L. Xu, et al., Chin. Chem. Lett. 30 (2019) 271-276. DOI:10.1016/j.cclet.2018.05.039 |
 2020, Vol. 31
2020, Vol. 31 

