b Research Institute of Sun Yat-sen University in Shenzhen, Shenzhen 518057, China;
c College of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, China
The boosted number of invalids suffered from bone defects caused by trauma or disease urged for new effective bone regeneration strategies [1]. It is well known that natural bone could hold excellent mechanical performance with complex functionalities which remains challenges to mimic [2, 3]. Thus, the gold standard for the materials utilized in clinical bone regeneration would still be autologous bone grafts with outstanding osteogenesis performance, however, limited by the availability and secondary injury [3]. With similar composition and structures, bone allografts have been available in orthopedic procedures though faced with reduced osteoinductivity and the risk of infection [4]. Hence, more and more researches focused on constructing scaffolds with different strategies to enhance bone regeneration [5-7]. To date, advances have been made in the development of different kinds of scaffolds made from synthetic materials, natural materials, or a combination of them, especially the scaffolds with biomimetic strategies for enhanced bone tissue engineering [8-10]. Nevertheless, the current scaffolds still exist a distance from ideal scaffolds for clinical use, urging for more exploration of new materials that could be fabricated as excellent scaffolds for potential bone regeneration.
As a source of nutrients in the diet, tofu prepared by traditional solidification holds varied protein, minerals and vitamins, in which the soy protein contains almost all essential amino acids with good biodegradability and biocompatibility as a promising substitute for tissue engineering while the calcium and magnesium could play a primary role in bone regeneration [11-13]. Structurally, the natural gel-like tofu possesses a natural porous architecture, suggesting good potential as a three-dimensional (3D) scaffold. Moreover, recent studies have proved the potential of tofu for biomedical applications with good biological safety [14]. With simple surface modification, the function of tofu scaffold could be further improved, indicating promising application potentiality in tissue engineering. Nevertheless, rare studies have utilized tofu as the natural scaffold for bone tissue engineering.
Hereon, wef abricated the tofu-based scaffolds and systematically explored the potential of tofu-based scaffolds for enhanced bone regeneration. In addition to the simple natural tofu scaffold, the surface modification with collagen has been made to furtherimprove the surface compatibility of the scaffold, since type I collagen fibrils could be the major organic polymers in the bone matrix, benefiting bone formation and remodeling [15, 16]. In our studies, the microstructure, mechanical properties, cytocompatibility, immunogenicity as well as bone regeneration ability of both tofu scaffolds and collagen-coated tofu scaffolds have been systematically investigated. The results showed that the tofu-based scaffolds with porous structure could support the adhesion and growth of human dental pulp stem cells (hDPSCs), suggesting good cytocompatibility. Notably, the tofu-based scaffolds could influence macrophages and promote the expression of osteogenesis-related genes and proteins, resulting in better bone regeneration after 2 months of implantation. All the results suggested that tofu as a natural sustainable porous scaffold could be a promising material with excellent bioactivities and environmental importance for potential bone regeneration.
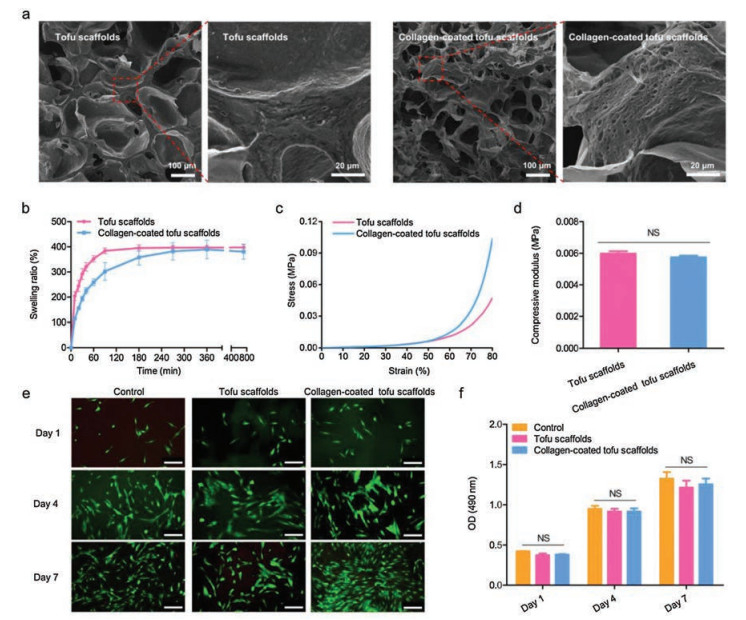
The tofu-based scaffolds were prepared by the conventional method according to the previous studies, and more details could be found in Supporting information [14]. The tofu scaffold could be milky white and brittle after lyophilization. The collagen sponge can be seen on the surface of the collagen-coated tofu scaffold. It is well known that scaffold materials utilized in bone tissue engineering need to support cell adhesion and growth, which could be partly depended on the structure and hygroscopicity of the scaffolds. Therefore, the internal morphology and swelling ratio of the tofu-based scaffolds should be confirmed. Scanning electron microscope (SEM) images revealed that the tofu-based scaffold possessed a porous microstructure (Fig. 1a). The pore sizes could be suitable for the growth of cells, and the connectivity of the pores would be conducive to the transportation of nutrients [17]. It is worth noting that there were some tiny pores on the pore wall of the tofu, which can still be observed after coating with the collagen, which may benefit the maintenance of water and nutrients and provide a good environment for cell growth. Comparing with the tofu scaffold without collagen, the addition of collagen resulted in a slight decrease in the initial swelling rates, but there was no significant difference in the final equilibrium swelling ratio between the two groups (Fig. 1b). Since the scaffolds need to be replaced in the defects under continuous compression during bone regeneration, the tissue engineering scaffold was supposed to possess specific mechanical properties. Here, the mechanical properties of the tofu-based scaffolds have been determined (Figs. 1c and d). The stress-strain curve demonstrated that the collagen-coated tofu scaffold may be able to withstand greater loads than the tofu scaffold, though there was no significant difference in the compressive modulus of the two groups, suggesting that the mechanical properties of the tofu-based scaffolds could be improved by further surface modification. All the results showed that the tofu-based scaffolds possessed proper pore structure and hygroscopicity, while the introduced collagen had no obvious influence on the scaffold architecture. Both the tofu scaffold and collagen-coated tofu scaffold would be suitable for bone tissue engineering.
|
Download:
|
| Fig. 1. Characterization and cytocompatibility of tofu-based scaffolds. (a) The interior morphology of tofu-based scaffolds. (b) Swelling kinetics of tofu scaffolds. (c, d) Mechanical properties of tofu scaffolds. (e) The live/dead staining assay. Scale bar: 100 μm. (f) MTT assay. | |
During the bone regeneration process, the interaction between different kinds of cells and the specific scaffolds would obviously affect the result. An ideal scaffold should have good cytocompatibility to support cell adhesion, growth, proliferation and even differentiation. To confirm the cytocompatibility of tofu-based scaffolds, live/dead viability assays and MTT assays were executed. The fluorescence images in live/dead viability assays confirmed the cell morphologies during the coculture. According to the results, the hDPSCs cocultured with tofu-based scaffolds could be elongated spindle, presenting good cell morphologies with rare red fluorescence (Fig. 1e). The results of MTT assays showed no significant difference in cell viability between each group (Fig. 1f), demonstrating that the tofu-based scaffolds could sustain the cell proliferation. Remarkedly, the group with collagen-coated tofu scaffolds indicated increased cell number though with on significant difference, which may due to the addition of collagen. Previous studies have found that collagen could improve cell adhesion and proliferation, as well as the cell survival under stress [18]. The coated collagen in the tofu-based scaffolds could help cells to adapt the microenvironment of tofu-based scaffolds and benefit the subsequent interaction between cells and scaffolds. All these results demonstrated that the tofu-based scaffolds possess satisfactory cytocompatibility with promising potential to be utilized in bone tissue engineering.
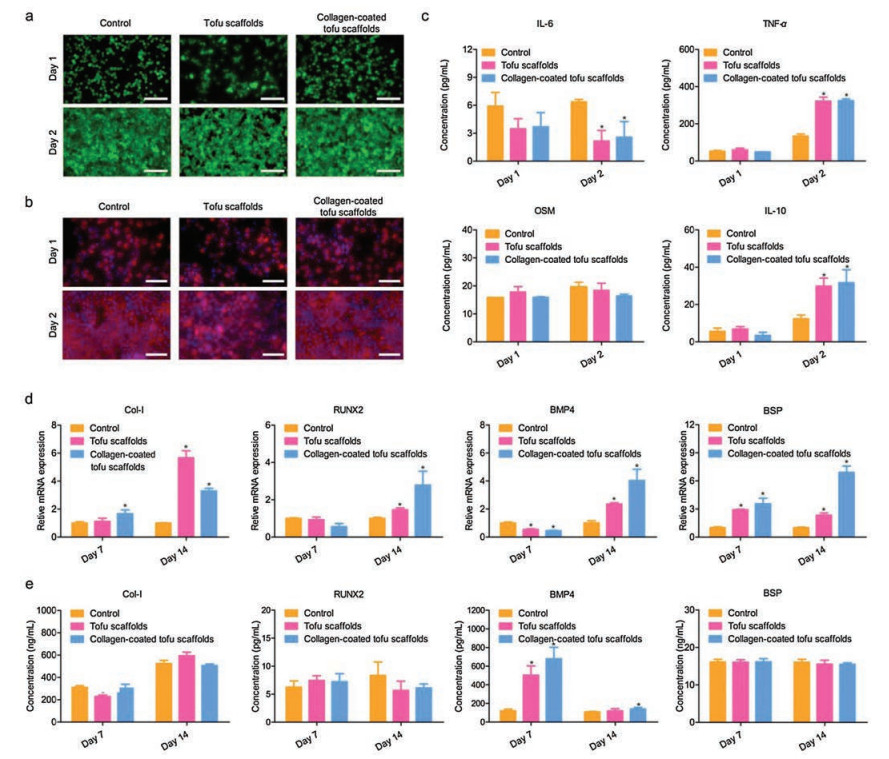
Meanwhile, the raw 264.7 cell as one of representative immune cells was utilized to investigate the immune responses caused by tofu-based scaffolds. According to the live/dead viability assays, most of the macrophages presented round shapes with green fluorescence, showing no significant differences between each group (Fig. 2a). After 2-day incubation, the number of raw 264.7 cells increased distinctly. On day 1, the cells growing on the surface of tofu scaffolds showed a trend of aggregation while the cells on the collagen-coated tofu scaffolds were almost evenly distributed. The aggregation of cells could be caused by the natural pores in tofu, and the coating of collagen could make the surface of the scaffold smooth and benefit the cells adhesion. On day 2, with good proliferation, the number of cells was enough to fill the natural pores on the surface of the tofu-based scaffolds with a trend to migrate into the scaffold. The stained cells in the fluorescence images further confirmed the cell morphology and proliferation, suggesting good cytocompatibility of the tofu-based scaffolds (Fig. 2b). Remarkably, though most cells presented round shapes in the group treated with tofu-based scaffolds, a few of cells on the tofu-based scaffolds had a trend to become elongated and showed more pseudopodia. According to the previous studies, shapes of macrophages could be related to distinct immune responses [19]. To further confirmed that, the releases of interleukin-6 (IL-6), Oncostatin M (OSM), tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10) in macrophages co-cultured with tofu-based scaffolds were confirmed by enzyme-linked immunosorbent assay (ELISA). The concentration of the IL-6 decreased on day 2 after treating with tofu-based scaffolds while the expression of OSM presented no significant difference between tofu-based scaffolds groups and control group (Fig. 2c). Meanwhile, the concentrations of the TNF-α and IL-10 showed significant increase on day 2 while being treated with tofu-based scaffolds. In addition, the expression of cytokines IL-6, OSM, TNF-α and IL-10 showed no significant difference between the group with tofu scaffolds and the group with collagen-coated tofu scaffolds.
|
Download:
|
| Fig. 2. Macrophage responses and in vitro osteogenic differentiation of hDPSCs co-cultured with tofu-based scaffolds. (a) The live/dead staining assay. Scale bar: 100 μm. (b) Morphology of RAW cells co-cultured with tofu-based scaffolds. Scale bar: 50 μm. (c) The release of IL-6, OSM, TNF-α and IL-10 in cells treated with tofu-based scaffolds. (d) Gene expression of Col-I, RUNX2, BMP4 and BSP in hDPSCs was evaluated with RT-qPCR and the data was normalized to GAPDH expression. (e) Osteogenic protein concentrations in the culture medium were measured by ELISAs. *P < 0.05 vs. control group. | |
To the best of our knowledge, inflammation could play an essential role in the early stage of bone repair [20]. Indeed, in the first few days of bone injury, hematomas could be formed in the defect site with infiltration of inflammatory cells and secretion of cytokines such as IL-6, OSM and TNF-α [21]. In human bodies, IL-6 could be involved in not only inflammation mediated by classic signaling, but also regenerationwith anti-inflammatory responses, which is mediated by trans-signaling [22]. In macrophage, the secreted IL-6 together with TNF-α as the typical pro-inflammatory cytokines could negatively impact the treatment of implanted scaffolds [23, 24]. In contrast, OSM, one of the cytokine members in classical IL-6 family related to in homeostasis and chronic inflammation, has been proved to support bone formation by signaling with OSM receptor (OSMR) and Stat3 [25, 26]. For bone regeneration, the inflammation should be controlled and eventually resolved. The anti-inflammatory cytokines like IL-10 could be the keys to achieve a balance between inflammation and regeneration [27]. Indeed, the IL-10 could be essential for the bidirectional regulation between inflammation and new bone formation [28]. It should be noticed that the macrophage phenotypes could be the plastic and dynamic with the release of different kinds of cytokines to respond to the environmental stimulation and perform a balance between inflammation and bone formation during the early bone regeneration process [29]. Hereon, the tofu-based scaffolds led to decreased IL-6 and increased IL-10 in the macrophages after incubation for 2 days, indicating the potential microenvironment for bone regeneration.
The good cytocompatibility and encouraging macrophage responses inspired the further investigation of bone regeneration potential of tofu-based scaffolds. To determine the osteogenic behaviors of cells treated with tofu-based scaffolds, the quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) and ELISA were employed to confirm the expression of osteogenesis-related genes and proteins including runt-related transcription factor 2 (RUNX2), bone morphogenic protein 4 (BMP4), type I pro-collagen (Col-I) and bone sialoprotein (BSP). According to previous studies, Col-I could benefit bone formation as a key between extracellular matrix and cells [30]. As a critical transcription factor for osteogenic differentiation, RUNX2 would be primary in bone regeneration and could be induced by BMP, which could make a difference to the expression of bone matrix proteins [31, 32]. The BSP could induce osteoblasts differentiation and benefit the calcium phosphate deposition with the association with Col-I during bone regeneration [33]. The results of RT-qPCR showed that the hDPSCs treated with tofu-based scaffolds presented an increased expression of Col-I, RUNX2, BMP4 and BSP after 14 days, indicating the promoted osteogenic differentiation (Fig. 2d). Meanwhile, the results of ELISAs further confirmed the presence of corresponding osteogenic differentiation-related proteins (Fig. 2e). The in vitro results have demonstrated that tofubased scaffolds could promote osteogenic differentiation, suggesting good potential for bone regeneration.
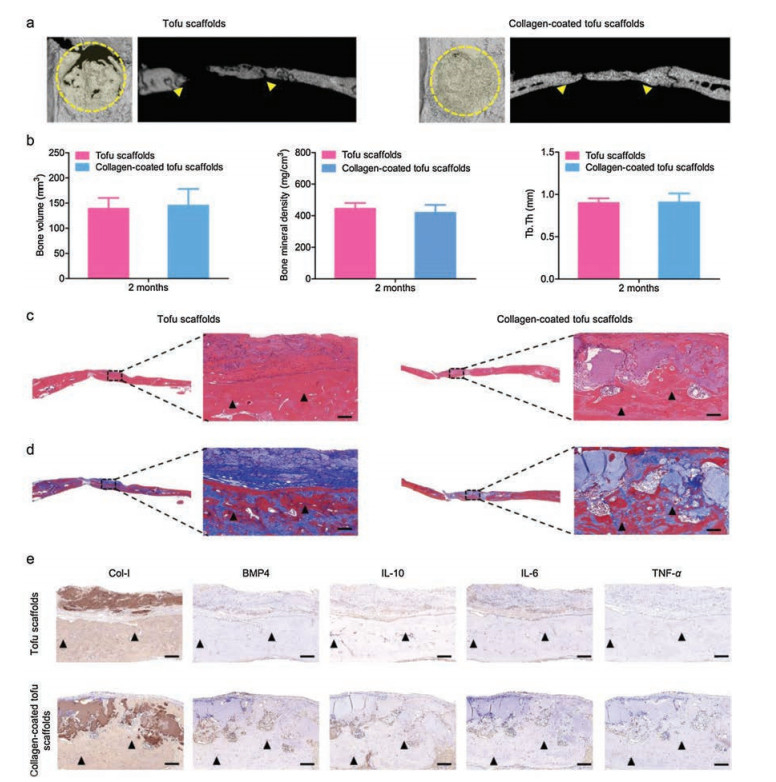
To further demonstrate the bone regeneration capability of tofu-based scaffolds in vivo, samples have been implanted in the bone defects of SD rats for 2 months. The CT images showed that abundant new-formed bones were presented in the defects after treating with tofu-based scaffolds for 2 months (Fig. 3a). Especially, the whole defects were the practically covered with new-formed bones after treated with collagen-coated tofu scaffolds. The statistics analysis further confirmed the bone mineral density, bone volume, and trabecular thickness (Tb.Th) of the newly formed bone (Fig. 3b). Moreover, histological analysis was performed to further investigate the new-formed bone tissues in the defects (Figs. 3c and d). It could be found that both the group treated with tofu scaffolds and the group treated with collagencoated tofu scaffolds presented abundant mature bone tissues with new blood vessel proliferated. Notably, the tofu scaffold with the collagen coating possessed improved surface compatibility. In addition, the results of immunohistochemical analysis showed the presence of Col-I, which could be essential for new bone formation (Fig. 3e) [30]. Meanwhile, the immunolabeling of IL-10 could be detected around bone defect, while little IL-6 and TNF-α could be found, suggesting a good microenvironment for bone regeneration with little inflammation. All the in vivo results demonstrated that the tofu-based scaffolds have good potential for bone regeneration.
|
Download:
|
| Fig. 3. Bone regeneration after 2-month implantation. (a) Representative micro-CT images of bone defects. (b) Quantitative analysis of bone miner density, bone volume and Tb.Th. (c, d) H&E and Masson's trichrome staining for new bone formation. (e) Immunohistochemistry of Col-I, BMP4, anti-inflammatory protein (IL-10) and proinflammatory proteins (IL-6 and TNF-α) after 2 months implantation. Triangle: new bone. Scale bar: 300 μm. | |
In this work, the tofu-based scaffolds were developed and systematically explored for enhanced bone regeneration. The surface modification of tofu scaffolds has been made with collagen by simple coating to further improve the surface compatibility during bone regeneration. The tofu-based scaffolds possessed porous structure and good cytocompatibility to support cell adhesion and growth. Remarkably, the tofu-based scaffolds could also influence macrophages and promote the expression of osteogenesis-related genes and proteins. With the surface modification by collagen, the tofu scaffold possessed improved surface compatibility and showed better bone regeneration after 2 months of implantation. All the results suggest that tofu as a sustainable natural porous scaffold would possess good potential for improving bone regeneration with proper modification.
Declaration of competing interestThe authors confirm that no conflicts of interest exist regarding the content of this article.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 51503129), Guangdong Innovative and Entrepreneurial Research Team Program (No. 2016ZT06S029), the funding from the Science and Technology Program of Guangzhou (No. 201707010094) and Science and Technology Planning Project of Shenzhen (No. JCYJ20180307163534533).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.002.
| [1] |
Y. Lai, Y. Li, H. Cao, et al., Biomaterials 197 (2019) 207-219. DOI:10.1016/j.biomaterials.2019.01.013 |
| [2] |
Z. Hao, Z. Song, J. Huang, et al., Biomater. Sci. 5 (2017) 1382-1392. DOI:10.1039/C7BM00146K |
| [3] |
H.C. Pape, A. Evans, P. Kobbe, J. Orthop. Trauma 24 (2010) S36-S40. DOI:10.1097/BOT.0b013e3181cec4a1 |
| [4] |
N.A. Beckmann, S. Mueller, M. Gondan, et al., J. Arthroplasty 30 (2015) 249-253. DOI:10.1016/j.arth.2014.09.016 |
| [5] |
C. Xian, Q. Yuan, Z. Bao, G. Liu, J. Wu, Chin. Chem. Lett. 31 (2020) 19-27. DOI:10.1016/j.cclet.2019.03.052 |
| [6] |
J.M. Holzwarth, P.X. Ma, Biomaterials 32 (2011) 9622-9629. DOI:10.1016/j.biomaterials.2011.09.009 |
| [7] |
G. Liu, Z. Bao, J. Wu, Chin. Chem. Lett. 31 (2020) 1817-1821. DOI:10.1016/j.cclet.2020.03.005 |
| [8] |
K. Huang, Z. Gu, J. Wu, ACS Biomater.-Sci. Eng. 6 (2020) 3037-3045. DOI:10.1021/acsbiomaterials.9b01997 |
| [9] |
H. Ren, Y. Cui, A. Li, D. Qiu, Chin. Chem. Lett. 29 (2018) 395-398. DOI:10.1016/j.cclet.2018.01.023 |
| [10] |
K. Huang, J. Wu, Z. Gu, ACS Appl. Mater. Inter. 11 (2018) 2908-2916. |
| [11] |
S. Ahn, C.O. Chantre, A.R. Gannon, et al., Adv. Healthc. Mater. 7 (2018) 1701175. DOI:10.1002/adhm.201701175 |
| [12] |
C.W. Xiao, J. Nutr. 138 (2008) 1244S-1249S. DOI:10.1093/jn/138.6.1244S |
| [13] |
D.L. Alekel, A.S. Germain, C.T. Peterson, et al., Am. J. Clin. Nutr. 72 (2000) 844-852. DOI:10.1093/ajcn/72.3.844 |
| [14] |
J. Huang, K. Huang, X. You, et al., J. Mater. Chem. B 6 (2018) 1328-1334. DOI:10.1039/C7TB02852K |
| [15] |
S.Q. Gong, Z.J. Xue, S.T. Liao, Y.B. Wu, Y. Liu, Chin. Chem. Lett. 29 (2018) 205-208. DOI:10.1016/j.cclet.2017.08.036 |
| [16] |
F. Nudelman, K. Pieterse, A. George, et al., Nat. Mater. 9 (2010) 1004-1009. DOI:10.1038/nmat2875 |
| [17] |
C.M. Murphy, M.G. Haugh, F.J. O'brien, Biomaterials 31 (2010) 461-466. DOI:10.1016/j.biomaterials.2009.09.063 |
| [18] |
C. Somaiah, A. Kumar, D. Mawrie, et al., PLoS One 10 (2015) e0145068. DOI:10.1371/journal.pone.0145068 |
| [19] |
Z. Chen, S. Ni, S. Han, et al., Nanoscale 9 (2017) 706-718. DOI:10.1039/C6NR06421C |
| [20] |
R. Dimitriou, E. Tsiridis, P.V. Giannoudis, Injury 36 (2005) 1392-1404. DOI:10.1016/j.injury.2005.07.019 |
| [21] |
P.K. Wong, I.K. Campbell, P.J. Egan, M. Ernst, I.P. Wicks, Arthritis Rheumatol. 48 (2003) 1177-1189. DOI:10.1002/art.10943 |
| [22] |
J. Scheller, A. Chalaris, D. Schmidt-Arras, S. Rose-John, BBA-Mol. Cell Res. 1813 (2011) 878-888. |
| [23] |
M. Murakami, D. Kamimura, T. Hirano, Immunity 50 (2019) 812-831. DOI:10.1016/j.immuni.2019.03.027 |
| [24] |
C. Xian, Z. Gu, G. Liu, J. Wu, Chin. Chem. Lett. 31 (2020) 1612-1615. DOI:10.1016/j.cclet.2019.09.011 |
| [25] |
P. Guihard, M.A. Boutet, B. Brounais-Le Royer, et al., Am. J. Clin. Pathol. 185 (2015) 765-775. DOI:10.1016/j.ajpath.2014.11.008 |
| [26] |
C.D. Richards, ISRN Inflammation 2013 (2013) 512103.
|
| [27] |
F. Deschaseaux, L. Sensébé, D. Heymann, Trends Mol. Med. 15 (2009) 417-429. DOI:10.1016/j.molmed.2009.07.002 |
| [28] |
B. Deng, M. Wehling-Henricks, S.A. Villalta, Y. Wang, J.G. Tidball, J. Immunol. 189 (2012) 3669-3680. DOI:10.4049/jimmunol.1103180 |
| [29] |
Z. Chen, T. Klein, R.Z. Murray, et al., Mater. Today 19 (2016) 304-321. DOI:10.1016/j.mattod.2015.11.004 |
| [30] |
A.M. Ferreira, P. Gentile, V. Chiono, G. Ciardelli, Acta Biomater. 8 (2012) 3191-3200. DOI:10.1016/j.actbio.2012.06.014 |
| [31] |
Y. Zhou, Y. Wu, X. Jiang, et al., PLoS One 10 (2015) e0129605.
|
| [32] |
H. Peng, V. Wright, A. Usas, et al., J. Clin. Invest. 110 (2002) 751-759. DOI:10.1172/JCI15153 |
| [33] |
S. Gomes, I.B. Leonor, J.F. Mano, R.L. Reis, D.L. Kaplan, Soft Matter 7 (2011) 4964-4973. DOI:10.1039/c1sm05024a |
 2020, Vol. 31
2020, Vol. 31 

