b Drug Discovery Research Center, Key Laboratory of Medical Electrophysiology of Ministry of Education, Southwest Medical University, Luzhou 646000, China
Infectious diseases caused by multidrug resistant bacteria have become one of the greatest threats to global public health [1]. Conventional antibiotics often appear to be incapable of responding to the prevalence of multidrug-resistant bacteria, either ineffective or inducing the emergence of new resistance after a period of use [2]. In this regard, engineered nanomaterials possess great advantages, which have attracted significantly attention as one of the most promising antimicrobial agents due to their exceptional antibacterial capacity, either inherent or chemically incorporated [3]. In particular, engineered nanostructures, including metals [4-8], metal oxides [9-11], polymers [12], and carbonbased (e.g., graphenes, carbon nanotubes and carbon quantum dots) [13-15] nanomaterials, have been proposed as potential antibacterial candidates to overcome multidrug resistant bacterial infections. Bacteria are also difficult to develop resistance to these antimicrobial nanomaterials due to their complex mechanism of action. However, although elemental nanostructures, such as silver nanoparticles (NPs) and graphene, possess an intrinsic broadspectrum antibacterial activity, they also present a serious threat due to their toxicity to mammalian cells [16, 17]. Therefore, it is significantly important to develop nanomaterials with intrinsically potent antibacterial activity but with an honest biocompatibility, as feasible candidates for much safer nanoantibiotics.
Gold, the "noblest" among the metals, is considered a biosafety metal element [18]. As expected, gold-based nanomaterials have also been demonstrated to possess superior biocompatibility in mammalian system, both in vitro and in vivo [19]. Benefit from their excellent biocompatibility as well as unusual physicochemical properties, gold nanomaterials have attracted significantly attention in biomedical applications such as bioanalysis, biocatalysis, bioimaging, drug delivery, and cancer therapy [20-22]. Recent several studies have also revealed the antibacterial properties of Au-based nanostructures [23-25]. These antibacterial strategies mainly rely on Au NPs as drug carriers to obtain antibacterial ability by grafting known antibacterial substances, such as cationic or zwitterionic ligands [24], antimicrobial peptides [26], and conventional small molecule antibiotics [27] on their surfaces. For example, Rotello's group found that zwitterionic Au NPs showed potent antimicrobial activity by tuning their sizes and ligand structures without visible cytotoxicity to human cells [28]. In addition, it has been found that Au NPs can also obtain antibacterial activity by adjusting their sizes or metal dopants [6, 29, 30].
On the other hand, the toxicological responses of nanomaterials are usually closely related to their structural and physicochemical properties [31]. Establishment of a structure-activity relationship for nanomaterials at the nano-bio interface is of great significance for deep understanding the toxic as well as antibacterial mechanisms of nanomaterials, enabling the circumvention of potential hazards and rational design of safer antibacterial nanomaterials [32]. The crystallographic facet is one of the most important aspects affecting the properties of nanomaterials [33], and the crystallographic facet exposures of nanomaterials often significantly influence their performance in optical, catalytic, and electrochemical properties [34-36]. Several studies have also demonstrated the interaction between crystallographic facets of nanomaterials (TiO2, ZnO, PbS, and Cu2O) with biological systems [33, 37, 38]. However, few studies have focused on the antibacterial behaviors and structure-activity relationships associated with well-defined crystallographic facets, especially for gold nanostructures.
In the present study, we report the synthesis of Au nanocrystals with different exposed facets to tune their antibacterial behaviors against the model bacteria Staphylococcus aureus for the first time. Nanocrystals with approximately the same total surface area were employed to compare the effect of different facets. We find that Au nanocrystals display substantial facet-dependent antibacterial activities. Although the high-index facets were considered to have higher surface energy as compared with low-index facets [39], neither of the two Au nanocrystals with high-index facets (i.e., {221}-facet trisoctahedra and {720}-facet concave cubes) exhibit antibacterial activity, whereas all the low-index facets (i.e., {100}-facet cubes, {110}-facet rhombic dodecahedra and {111}-facet octahedra) exhibit considerable antibacterial activity. Toxicity mechanism studies have shown that the facet-dependent antibacterial activities of Au nanocrystals mainly caused by differential bacterial membrane damage as well as inhibition of protease activity and energy metabolism, instead of production of reactive oxygen species (ROS) by most antibiotics as well as antimicrobial nanostructures [40, 41].
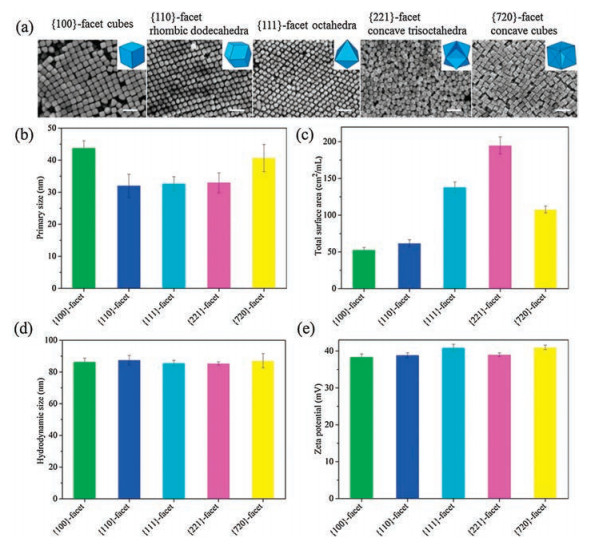
Au nanocrystals were prepared through a seed-mediated growth protocol using HAuCl4 as precursors in the presence of cetyltrimethylammonium chloride (CTAC) surfactant. Trace amounts of NaBr was introduced in the formation of nanocubes with {100} facets, rhombic dodecahedra with {110} facets and trisoctahedra with {221} facets, while a trace amount of KI and AgNO3 was added in the synthesis of octahedra with {111} facets and concave nanocubes with {720} facets, respectively. The scanning electron microscope (SEM) images indicate that the prepared Au nanocrystals display cubic, rhombic dodecahedral, octahedral, trisoctahedral, and concave cubic geometries (Fig. 1a). Here the X-ray diffraction (XRD) patterns also clear confirmed that we successfully prepared the Au nanocrystals with designed crystal form (Fig. S1 in Supporting information), corresponding to those reported in previous study [42-44]. The edge lengths of these Au nanocrystals were determined at the range of 32–44 nm (Fig. 1b), and their specific surface areas were calculated at the range of 53–195 cm2/mL (Fig. 1c). All Au nanocrystals were well dispersed in water, resulting in hydrodynamic sizes of 85–88 nm (Fig. 1d). The zeta potentials range from 38 mV to 41 mV indicating parallel positive charges on the surface of nanocrystals (Fig. 1e). Furthermore, the thermogravimetric (TG) analysis showed that there is no obvious weight-loss stage at temperatures up to 800 ℃ for these Au nanocrystals under N2 conditions, indicating that only small amounts of CTAC are present on the surface of nanocrystals (Fig. S2 in Supporting information). Overall, these analyses show that the differences between various Au nanocrystals are mainly attributed to the surface facets.
|
Download:
|
| Fig. 1. Characterization of Au nanocrystals. (a) SEM images. Scale bar: 100 nm. (b) Primary sizes. (c) Specific surface area. (d) Hydrodynamic sizes. (e) Zeta potentials in water. | |
For accurate comparison of antibacterial activity, the total surface area of different particles should be uniform. To facilitate the comparison, the total particle weight in 1000 μL of the nanocrystal solution was firstly determined for each sample (Table S1 in Supporting information). According to the particle edge lengths shown in SEM images, the volume, mass and total surface area of a single nanocrystal can be calculated. Eventually, different volumes of the nanocrystal solutions with same total particle surface area were used for the following antibacterial activity tests.
To compare the antibacterial properties of Au nanocrystals with different crystallographic facets, a series of antibacterial assays were performed using S. aureus as a representative pathogen. First, we performed the agar diffusion method to visually compare the antibacterial abilities of these Au nanocrystals, as illustrated in Fig. 2a. At the same concentration (20 cm2/mL), the groups treated with low-index facets Au nanocrystals, i.e., {100}-facet cubes, {110}-facet rhombic dodecahedra, and {111}-facet octahedral, showed clearly transparent and significantly enhanced inhibition zone compared to that of the {221}-facet trisoctahedra group and {720}-facet concave cubes group, two Au nanocrystals with high-index facets, demonstrating the superior antibacterial capacity of nanocrystals with low-index facets. Among them, the inhibition zone of {110}-facet Au nanocrystals treatment group is the most significant, indicating that it may have the strongest antibacterial activity. In addition, CTAC of 100 μg/mL as a control treatment also showed no visible inhibition zone, indicating that the different antibacterial activities were attributed to the different crystallographic facets configurations on the Au nanocrystals, regardless of the small amount of CTAC remaining on the surface.

|
Download:
|
| Fig. 2. Antibacterial properties of Au nanocrystals with different crystallographic facets. (a) Inhibition zone measurements by agar diffusion method. (b) Bacterial relative growth after treatment with water, {100}-facet cubes, {110}-facet rhombic dodecahedra, {111}-facet octahedra, {221}-facet trisoctahedra, and {720}-facet concave cubes. Data are means±SD, n = 3. (c) The MICs of Au nanocrystals against S. aureus. | |
Furthermore, we quantitatively compared the survival of bacteria after treatment of different Au nanocrystals at different times. As shown in Fig. 2b, compared with the control group, the bacterial relative growth was inhibited to various degrees after treatment with Au nanocrystals for 1 h. After 2 h of treatment, the number of bacteria surviving in the {100}, {110}, and {111} Au nanocrystals treatment group was further reduced, and the {110}-facet rhombic dodecahedra was the most effective. In contrast, the number of bacteria in the {221}-facet trisoctahedra group and {720}-facet concave cubes group began to increase, showing no visible bacteriostatic effect (Fig. 2b). The minimum inhibitory concentration (MIC) measurements showed that the MIC of {100}-facet cubes, {110}-facet rhombic dodecahedra, and {111}-facet octahedral were 1, 0.5, and 8 cm2/mL, respectively, and the MIC of {221}-facet trisoctahedra and {720}-facet concave cubes were greater than 32 cm2/mL (Fig. 2c). Combined with the above results, it can be confirmed that in the Au nanocrystals with different crystallographic facets configurations, the {110}-facet rhombic dodecahedra shows the highest antibacterial activity, followed by {100} and {111} Au nanocrystals, and {221}-facet trisoctahedra and {720}-facet concave cubes basically showed no antibacterial activity. These results also indicate that the gold nanomaterials possess facet-dependent antibacterial activity, and the crystal face configuration significantly affects the antibacterial properties of the gold nanomaterials.
To further study the facet-dependent antibacterial properties of Au nanocrystals, we employed a LIVE/DEAD bacterial viability staining kit to visualize the damage of bacterial cell membranes by Au nanocrystals through a confocal microscope. As shown in Fig. 3a, the treatment by {100}-facet cubes, {110}-facet rhombic dodecahedra, and {111}-facet octahedra lead to severe cell membrane damage, as evidenced by the strong red fluorescence emission of intracellular propidium iodide (PI) after binding with nucleic acids. In particular, the group treated with {110}-facet rhombic dodecahedra barely show the green fluorescence, corresponding to the strongest bactericidal effects. In contrast, the {221}-facet trisoctahedra and {720}-facet concave cubes treatment groups were similar to the control group (watertreated), with almost only green fluorescence, demonstrating that the bacterial cell structures had not been destroyed (Fig. 3a). Moreover, the SEM images revealed different degrees of membrane damage in Au nanocrystals-treated S. aureus. Unlike the intact and smooth morphology of S. aureus cells after treatment in water, {221}-facet trisoctahedra and {720}-facet concave cubes, the lowindexed Au nanocrystals with {100}-facet cubes, {110}-facet rhombic dodecahedra, and {111}-facet octahedra induce significant cell damage and wizened (Fig. 3b).

|
Download:
|
| Fig. 3. Assessments of the S. aureus membrane integrity treated by Au nanocrystals. (a) Confocal laser scanning microscopy (CLSM) images of S. aureus treated with Au nanocrystals. The dead bacteria were visualized by PI staining (red fluorescence), while the SYTO 9 (green fluorescence) helped to identify all cells. Scale bar: 30 μm. (b) SEM micrographs of S. aureus treated with water, {100}-facet cubes, {110}-facet rhombic dodecahedra, {111}-facet octahedral, {221}-facet trisoctahedra and {720}-facet concave cubes (from top to bottom), respectively. Scale bar: 2 μm. (c) DNA release of S. aureus treated with Au nanocrystals. * means statistically significant difference from control water group (P < 0.05). (d) LDH release of S. aureus treated with Au nanocrystals. * means statistically significant difference from {720}-facet concave cubes group (P < 0.05). | |
We further examined the close relationship between the facetdependent antibacterial activity of Au nanocrystals and bacterial membrane damage by performing nucleic acids leakage and lactate dehydrogenase (LDH) release assays. Several works proposed that the release of intracellular components may serve as useful markers of cell membrane integrity [45-47]. The amount of DNA released from bacterial nucleic acids can be determined by recording the optical density at 260 nm (OD260). Compared to the control treatment, the amount of DNA released from S. aureus remarkably increased upon treatment with Au nanocrystals with {100}-facet cubes, {110}-facet rhombic dodecahedra, and {111}-facet octahedra, while no changes are found after treatment with Au nanocrystals with {221}-facet trisoctahedra and {720}-facet concave cubes (Fig. 3c). LDH, as a vital regulator of cellular biochemical processes, is also a well-known indicator of bacterial membrane integrity [47]. Here the LDH release assays also demonstrated similar results. As shown in Fig. 3d, the {100}-facet cubes, {110}-facet rhombic dodecahedra, and {111}-facet octahedra treated groups exhibit significantly enhanced LDH release compared to {221}-facet trisoctahedra and {720}-facet concave cubes treated groups. These membrane integrity results were in good agreement with our antibacterial measurements in Fig. 2, suggesting that the facet-dependent antibacterial behavior of Au nanocrystals might be directly associated with the effective damage to the bacterial cell membrane.
Many antibiotics and antibacterial nanomedicine owe their antibacterial efficacy to their ability to modulate the ROS generation that eventually induces bacterial death [40, 41, 48]. As such, we attempt to clarify whether the facet-dependent antibacterial efficiency of Au nanocrystals was driven by ROS production. To investigate this point, we directly determined the level of ROS in bacterial cells after treated with the Au nanocrystals. As shown in Fig. S3 (Supporting information), we found that Au nanocrystals cannot observably increase the generation of total ROS according to the cellular staining by 20, 70-dichlorofluorescein diacetate (DCFH-DA). In comparison, a dramatic increase of intracellular ROS typically appears within 30 min for bactericidal antibiotics treated bacteria [40]. In addition, as one of the most important antioxidants in cells, the change in glutathione (GSH) content directly reflects the level of ROS oxidative stress injury in bacteria. As expected, the GSH content in S. aureus did not significantly decrease after treatment of Au nanocrystals (Fig. S4 in Supporting information). These results clearly suggest that the facet-dependent antibacterial activity of Au nanocrystals may not be involved in any ROS regulated processes, and the antibacterial mechanisms of Au nanocrystals are quite different from conventional nanoantibiotics, such as gold nanoclusters [29] and Pd polyhedral [7].
We further explored the antibacterial mechanism of Au nanocrystals. In addition to ROS induced oxidative damage, previous studies have also shown that the antibacterial activity by nanomedicine is also related to inhibition of bacteria metabolism. For example, Dai and coworkers found that the antibacterial mechanism of cationic polymers-capped Ag NPs is closely related to the inhibition of bacterial cell enzyme activities, in addition to inducing ROS generation [49]. Jiang and coworkers found that the antibacterial activity of AuPt bimetallic NPs is only related to their induced damage of cell membrane and influence of ATP synthesis, but not related to ROS [6]. A similar mechanism was also found for antibacterial behavior by cationic Au NPs [50]. Hence we hypothesized that the facet-dependent antibacterial properties of Au nanocrystals may also be related to disrupting the normal metabolism of S. aureus, such as inhibition of cellular enzyme activity and ATP synthesis.
To prove our hypothesis, we first measured the inhibitory effect of Au nanocrystals on β-galactosidase activity of S. aureus. β-Galactosidase was a marker enzyme used to evaluate whether the nanomedicine can inhibit the metabolic pathways [49]. The β-galactosidase activity can be evaluated by measuring orthonitrophenol (ONP) level because it can catalyze the hydrolysis of o-nitrophenyl-β-D-galactopyranoside (ONPG) to form ONP, a yellow chromogenic compound and can be evaluated by measuring the OD420. ONP production decreased when β-galactosidase activity was inhibited by the nanomedicine. As shown in Fig. 4a, 61.34%, 82.35%, and 68.07% of β-galactosidase was inhibited after the bacteria were treated with {100}-facet cubes, {110}-facet rhombic dodecahedra, and {111}-facet octahedral, respectively. In contrast, the inhibition ratio of {221}-facet trisoctahedra and {720}-facet concave cubes was only 10.08% and 16.81%, respectively. Clearly, the decrease in β-galactosidase activity varies with the facet of Au nanocrystals. Therefore, the inhibition of bacterial enzymatic activity is also one of the reasons for the facetdependent antibacterial behaviors by Au nanocrystals. In addition, since ATP plays a vital role in bacterial metabolism, we also measured the effect of Au nanocrystals on bacterial ATP (Fig. 4b). We found that the intracellular ATP levels of bacteria are strongly inhibited in {100}-facet cubes, {110}-facet rhombic dodecahedra, and {111}-facet octahedral treated groups, with only 7.60%, 1.57%, and 3.10% of the control group using only water, respectively. In comparison, the bacterial intracellular ATP levels are largely maintained for {221}-facet trisoctahedra (79.58%) and {720}-facet concave cubes (83.27%) treated group, respectively. Therefore, we can see that Au nanocrystals with {100}-facet cubes, {110}-facet rhombic dodecahedra, and {111}-facet octahedral may cause huge damage to bacterial enzyme activity and energy metabolic levels. These combined effects can lead to bacterial death. To summarize, Au nanocrystals exhibited facet-dependent antibacterial activity, which are mainly related to the induction of bacterial membrane damage, the inhibition of enzyme activity, and the loss of energy metabolism, but irrelevant to ROS generation (Fig. 4c).

|
Download:
|
| Fig. 4. Evaluations of the metabolic levels after treatment with Au nanocrystals in S. aureus. (a) The bacterial β-galactosidase activity treated with different facets of Au nanocrystals. (b) Intracellular ATP concentrations after treated with different facets of Au nanocrystals. The water treated group was used as controls. * means statistically significant difference from control water group (P < 0.05). (c) Schematic illustration of the facet regulation of Au nanocrystals to significantly affect their antibacterial properties. | |
In summary, the effect of the exposed facet of Au nanocrystals on the antibacterial activity was evaluated for the first time. Crystallographic facet exposures of nanomaterials could significantly influence their biomedical efficacy. This paradigm drives much nanomaterials-based therapeutics as the common belief that the high-index facets were considered to have higher bioactivity as compared with low-index facets [32, 33, 37, 39]. However, the presented work shows an opposite effect when employing faceted Au nanocrystals as a model to investigate the effect of crystallographic facet exposures on their antibacterial performance. Au nanocrystals display substantial facet-dependent antibacterial activities. The low-index facets of cubes, octahedra, and rhombic dodecahedra show considerable antibacterial activity, whereas the high-index facets of trisoctahedra and concave cubes remain inert under biological conditions. This result is in stark contrast to the previous paradigm. The antibacterial mechanism studies have shown that the facet-dependent antibacterial behaviors of Au nanocrystals are mainly caused by differential bacterial membrane damage as well as inhibition of cellular enzymatic activity and energy metabolism. The faceted Au nanocrystals are unique in that they do not induce generation of ROS, as validated for most antibiotics and antimicrobial nanostructures. The present study may provide a deeper understanding of facet-dependent toxicological responses, which might offer a new perspective for developing novel nanoantibiotics with enhanced antibacterial activity.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Key Research and Development Program of China (No. 2017YFA0205300), the National Natural Science Foundation of China (Nos. 21675023 and 91753106) and the Scientific Research Foundation of Southwest Medical University (No. 19120200037).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.05.035.
| [1] |
B.D. Lushniak, Public Health Rep. 129 (2014) 314-316. DOI:10.1177/003335491412900402 |
| [2] |
A.J. Huh, Y.J. Kwon, J. Control. Release 156 (2011) 128-145. DOI:10.1016/j.jconrel.2011.07.002 |
| [3] |
A. Gupta, S. Mumtaz, C.H. Li, I. Hussain, V.M. Rotello, Chem. Soc. Rev. 48 (2019) 415-427. DOI:10.1039/C7CS00748E |
| [4] |
J. Xia, W. Wang, X. Hai, et al., Chin. Chem. Lett. 30 (2019) 421-424. DOI:10.1016/j.cclet.2018.07.008 |
| [5] |
J. Gong, Y. Zhang, Y. Huang, et al., J. Biomed. Nanotechnol. 15 (2019) 2262-2270. DOI:10.1166/jbn.2019.2851 |
| [6] |
Y. Zhao, C. Ye, W. Liu, R. Chen, X. Jiang, Angew. Chem. Int. Ed. 53 (2014) 8127-8131. DOI:10.1002/anie.201401035 |
| [7] |
G. Fang, W. Li, X. Shen, et al., Nat. Commun. 9 (2018) 129.
|
| [8] |
S. George, I. Tay, W.H. Phue, H. Gardner, B. Sukumaran, J. Biomed. Nanotechnol. 15 (2019) 2216-2228. DOI:10.1166/jbn.2019.2858 |
| [9] |
H. Sun, Z. Yang, Y. Pu, et al., J. Colloid Interface Sci. 547 (2019) 40-49. DOI:10.1016/j.jcis.2019.03.061 |
| [10] |
K. Qi, B. Cheng, J. Yu, W. Ho, J. Alloys. Compd. 727 (2017) 792-820. DOI:10.1016/j.jallcom.2017.08.142 |
| [11] |
K. Qi, X. Xing, A. Zada, et al., Ceram. Int. 46 (2020) 1494-1502. DOI:10.1016/j.ceramint.2019.09.116 |
| [12] |
P. Xia, S. Cao, B. Zhu, et al., Angew. Chem. Int. Ed. 59 (2020) 5218-5225. DOI:10.1002/anie.201916012 |
| [13] |
H. Ji, H. Sun, X. Qu, Adv. Drug Deliver. Rev. 105 (2016) 176-189. DOI:10.1016/j.addr.2016.04.009 |
| [14] |
H. Zheng, R. Ma, M. Gao, et al., Sci. Bull. (Beijing) 63 (2018) 133-142. DOI:10.1016/j.scib.2017.12.012 |
| [15] |
H. Liu, X. Liu, F. Zhao, et al., J. Colloid Interface Sci. 562 (2020) 182-192. DOI:10.1016/j.jcis.2019.12.017 |
| [16] |
M.I. Setyawati, X. Yuan, J. Xie, D.T. Leong, Biomaterials 35 (2014) 6707-6715. DOI:10.1016/j.biomaterials.2014.05.007 |
| [17] |
K.H. Liao, Y.S. Lin, C.W. Macosko, C.L. Haynes, ACS Appl. Mater. Interfaces 3 (2011) 2607-2615. DOI:10.1021/am200428v |
| [18] |
B. Hammer, J.K. Norskov, Nature 376 (1995) 238.
|
| [19] |
N. Lewinski, V. Colvin, R. Drezek, Small 4 (2008) 26-49. DOI:10.1002/smll.200700595 |
| [20] |
J. Beik, M. Khateri, Z. Khosravi, et al., Coord. Chem. Rev. 387 (2019) 299-324. DOI:10.1016/j.ccr.2019.02.025 |
| [21] |
S. Yang, L. Zhou, Y. Su, R. Zhang, C.M. Dong, Chin. Chem.Lett. 30 (2019) 187-191. DOI:10.1016/j.cclet.2018.02.015 |
| [22] |
Y. Zheng, L. Lai, W. Liu, H. Jiang, X. Wang, Adv. Colloid Interface Sci. 242 (2017) 1-16. DOI:10.1016/j.cis.2017.02.005 |
| [23] |
C.D. Tran, F. Prosenc, M. Franko, J. Colloid Interface Sci. 510 (2018) 237-245. DOI:10.1016/j.jcis.2017.09.006 |
| [24] |
Y. Zhao, Y. Tian, Y. Cui, et al., J. Am. Chem. Soc. 132 (2010) 12349-12356. DOI:10.1021/ja1028843 |
| [25] |
X. Li, S.M. Robinson, A. Gupta, et al., ACS Nano 8 (2014) 10682-10686. DOI:10.1021/nn5042625 |
| [26] |
W.Y. Chen, H.Y. Chang, J.K. Lu, et al., Adv. Funct. Mater. 25 (2015) 7189-7199. DOI:10.1002/adfm.201503248 |
| [27] |
X. Yang, J. Yang, L. Wang, et al., ACS Nano 11 (2017) 5737-5745. DOI:10.1021/acsnano.7b01240 |
| [28] |
S. Huo, Y. Jiang, A. Gupta, et al., ACS Nano 10 (2016) 8732-8737. DOI:10.1021/acsnano.6b04207 |
| [29] |
K. Zheng, M.I. Setyawati, D.T. Leong, J. Xie, ACS Nano 11 (2017) 6904-6910. DOI:10.1021/acsnano.7b02035 |
| [30] |
Y. Zheng, W. Liu, Z. Qin, et al., Bioconjugate Chem. 29 (2018) 3094-3103. DOI:10.1021/acs.bioconjchem.8b00452 |
| [31] |
H. Johnston, D. Brown, A. Kermanizadeh, E. Gubbins, V. Stone, J. Control. Release 164 (2012) 307-313. DOI:10.1016/j.jconrel.2012.08.018 |
| [32] |
A. Nel, T. Xia, H. Meng, et al., Acc. Chem. Res. 46 (2013) 607-621. DOI:10.1021/ar300022h |
| [33] |
N. Liu, K. Li, X. Li, et al., ACS Nano 10 (2016) 6062-6073. DOI:10.1021/acsnano.6b01657 |
| [34] |
M.H. Huang, G. Naresh, H.S. Chen, ACS Appl. Mater. Interfaces 10 (2017) 4-15. |
| [35] |
C.Y. Chiu, P.J. Chung, K.U. Lao, C.W. Liao, M.H. Huang, J. Phys.Chem. C 116 (2012) 23757-23763. DOI:10.1021/jp307768h |
| [36] |
J. Liao, F. Yang, C.Z. Wang, S. Lin, Sens. Actuat. B:Chem. 271 (2018) 195-202. DOI:10.1016/j.snb.2018.05.067 |
| [37] |
Y. Chang, K. Li, Y. Feng, et al., Nano Res. 9 (2016) 3812-3827. DOI:10.1007/s12274-016-1251-2 |
| [38] |
J. Ren, W. Wang, S. Sun, et al., Ind. Eng. Chem. Res. 50 (2011) 10366-10369. DOI:10.1021/ie2005466 |
| [39] |
Z. Quan, Y. Wang, J. Fang, Acc. Chem. Res. 46 (2013) 191-202. DOI:10.1021/ar200293n |
| [40] |
M.A. Kohanski, D.J. Dwyer, B. Hayete, C.A. Lawrence, J.J. Collins, Cell 130 (2007) 797-810. DOI:10.1016/j.cell.2007.06.049 |
| [41] |
B. Yang, Y. Chen, J. Shi, Chem. Rev. 119 (2019) 4881-4985. DOI:10.1021/acs.chemrev.8b00626 |
| [42] |
H.L. Wu, C.H. Kuo, M.H. Huang, Langmuir 26 (2010) 12307-12313. DOI:10.1021/la1015065 |
| [43] |
J. Zhang, M.R. Langille, M.L. Personick, et al., J. Am. Chem. Soc. 132 (2010) 14012-14014. DOI:10.1021/ja106394k |
| [44] |
P.J. Chung, L.M. Lyu, M.H. Huang, Chem. Eur. J. 17 (2011) 9746-9752. DOI:10.1002/chem.201101155 |
| [45] |
C. Chen, S.L. Cooper, Biomaterials 23 (2002) 3359-3368. DOI:10.1016/S0142-9612(02)00036-4 |
| [46] |
S. Anbu, S. Kamalraj, B. Varghese, J. Muthumary, M. Kandaswamy, Inorg. Chem. 51 (2012) 5580-5592. DOI:10.1021/ic202451e |
| [47] |
W.K. Oh, S. Kim, H. Yoon, J. Jang, Small 6 (2010) 872-879. DOI:10.1002/smll.200902074 |
| [48] |
Y. Zheng, W. Liu, Y. Chen, et al., J. Colloid Interface Sci. 546 (2019) 1-10. DOI:10.1016/j.jcis.2019.03.052 |
| [49] |
X. Dai, X. Chen, J. Zhao, et al., ACS Appl. Mater. Interfaces 9 (2017) 13837-13848. DOI:10.1021/acsami.6b15821 |
| [50] |
Y. Cui, Y. Zhao, Y. Tian, et al., Biomaterials 33 (2012) 2327-2333. DOI:10.1016/j.biomaterials.2011.11.057 |
 2020, Vol. 31
2020, Vol. 31 

