b Department of Neurosurgery, First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, China
Tumor microenvironments have many characteristics different from those of normal tissues, such as acidity under anoxic conditions [1]. According to this pathological feature, a pH-sensitive polymeric drug-carrier system to target lesion sites could be prepared to increase the enrichment and tissue uptake of therapeutic molecules at tumor sites and improve the therapeutic effect of drugs currently available for clinical use. To develop pH-sensitive smart-response polymer materials for acidic tumor microenvironments, there are two principle strategies [2, 3]. First is the cleavage of acid-sensitive chemical bonds such as hydrazones to change the composition of polymer chain segments and affect the pH response [4]. In the second approach, ionizable or protonatable groups can be incorporated in the polymer chain segments [5]. For the condition in which different pH values exist on both sides of the isoelectric point, the charge properties of the chain segments change to realize a conformational response or potential of transmission structure to pH value [6]. For example, histidine contains an imidazole group with an isoelectric point pKa of about 6.9; this group can be protonatedas the environment changes from alkaline to acidic, thus improving the molecular charge from negative to positive [7]. Accordingly, numerous studies have shown that the solubility of polyhistidine changes with the protonation of its imidazole groups, and its chain segments change from hydrophobic to hydrophilic. The ABC triblock copolymer PLA-b-PEG-b-PLH (PLA, polylipoic acid; PEG, polyethylene glycol; PLH, poly-L-histidine) synthesized by Lee et al. exhibited pH sensitivity, forming micelles with flower-like configurations and fulfilling the requirements for the intelligent response of a drug carrier to the acidic tumor environment [8]. In summary, polyhistidine chain segments have become an essential chemical constituent unit in studies on the development of intelligent acid-responsive carriers.
Fluorescence resonance energy transfer (FRET) is a highly sensitive, high-spatial-resolution, and non-destructive homogeneous-phase detection technology that combines the fluorescence phenomenon with resonance energy transfer [9-11]. It is widely used in the field of analytical chemistry [12]. In FRET systems, when the fluorescence donor is physically close to the receptor (1–10 nm), the emission spectrum of the donor overlaps with the absorption spectrum of the receptor to some extent. Due to dipole-dipole interactions, the excited state energy of the donor is transferred to the receptor in a non-radiative form, resulting in the FRET effect. A drug-carrier model exhibiting the FRET effect would be able to regulate the imaging potential of an integrated diagnostic and treatment drug-loading system, as well as indicate the metabolism of the carrier or control the on/off switching of fluorescence signals [13, 14].
The amphiphilic block copolymer PCL-b-PEG-b-PCL is a well-known material with good biocompatibility [15]. It can realize the self-folding of its polymer chain segments and manifests as self-assembled nanoparticles or temperature-sensitive gels [16]. Despite these useful features, such polymer-carriers have neither pH sensitivity nor chemical handles for fluorescence labeling on their chain segments, so integrated diagnosis and treatment functions cannot be realized in vivo [17, 18].
In this study, we designed and synthesized a polyhistidine-b-polyethylene glycol-b-polycaprolactone (PLH-PEG-PCL) triblock copolymer to take advantage of its folding and assembly characteristics (Fig. 1). We chemically modified each end of the polymer: A fluorescent dye probe molecule was added as the donor and gold nanorods as the receiver was incorporated after introducing a linker, disulfide-bearing α-lipoic acid, at the C-terminus, thus obtaining Cy7-labeled PLH-PEG-PCL-LA/Au complexes. Through a dynamic self-assembly process, we constructed this FRET system by which to explore the microenvironmental adjustment of fluorescence.
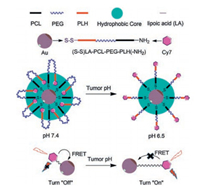
|
Download:
|
| Fig. 1. Schematic diagram of fluorescence regulation of pH-sensitive allosteric composite system based on the FRET principle. | |
The Fmoc-PEG-b-PCL diblock copolymer was synthesized by the ring-opening polymerization of Fmoc-PEG-OH and ε-CL catalyzed by Sn(Oct)2 [19]. The calculated amounts of Fmoc-PEG-OH and ε-CL based on a preset weight ratio were added to a dried glass ampoule; then, 0.5% w/w Sn(Oct)2 was added, and the mixture was heated to 135 ℃ with stirring under nitrogen atmosphere. After 6 h, DCM was used as the solvent and cold diethyl ether as the precipitant to precipitate the extracted product. Then, the obtained crude FmocPEG-b-PCL copolymer was dialyzed in distilled water for two days, freeze-dried, and stored in a desiccator.
The α-lipoic acid was used to modify the PCL segment. First, Fmoc-PEG-b-PCL (0.25 mol) and α-lipoic acid (0.5 mmol) were dissolved in DCM (25 mL). Then, DCC and DMAP (0.5 mmol) were added, and the solution was stirred at room temperature for 24 h. Fmoc-PEG-b-PCL-LA was collected by the cold diethyl ether coprecipitation method and dried under vacuum at room temperature.
Subsequently, the crude Fmoc-PEG-b-PCL-LA product (0.25 mmol) was dissolved in piperidine-DCM (1:4, v/v) solution and stirred for 10 min. The deprotected product (NH2)-PEG-b-PCL-LA was collected by diethyl ether coprecipitation and then dialyzed with distilled water for two days, after which the purified (NH2)- PEG-b-PCL-LA was lyophilized to constant weight.
The final copolymer, PLH-b-PEG-b-PCL-LA, was synthesized by ring-opening polymerization [20, 21]. First, Nα-Cbz-Nim-DNP-L-histidine (1 g) was dissolved in anhydrous THF (25 mL), and thionyl chloride (5 mL) was added dropwise while stirring. After the solution clarified, stirring was continued until the reaction was complete, ~1 h. The product was precipitated with anhydrous ether to obtain HisNCA. Next, the HisNCA (0.5 g) and freshly prepared NH2-PEG-b-PCL-LA (50 mg) were dissolved together in DMF (50 mL, contained 0.01 mmol RSH), allowed to react for 24 h, and precipitated with anhydrous ether to obtain PLH-PEG-b-PCL-LA. The PLH-b-PEG-b-PCL-LA powder obtained by the above method was dissolved in DMSO and dialyzed in deionized water using a dialysis bag (molecular weight cut-off 3000 Da) to get self-assembled nanostructures.
The FRET model was prepared used gold nanorods and Cy7- labled self-assembled nanoparticles. Firstly, aqueous CTAB (0.2 mol/L, 5 mL), HAuCl4 (0.5 mmol/L, 5 mL), and freshly prepared aqueous NaBH4 solution (10 mmol/L, 0.6 mL) were successively added to a flask to prepare the seed solution. In a different round-bottomed flask, AgNO3 solution (4 mmol/L, 1.5 mL), HAuCl4 (1 mmol/L, 50 mL), CTAB (0.2 mol/L, 50 mL), vitamin C (0.1 mol/L, 0.7 mL), and the seed solution (0.12 mL) were sequentially added, stirred at room temperature for 20 h, and centrifuged for later use [22]. Subsequently, Cy7-PLH-b-PEG-b-PCL-LA was dissolved in DMSO, and DTT (5 nmol/L) and gold nanorods (1 mg/mL) were added. After 6-h stirring, the construction of the polymernanogold complex FRET model was completed.
The FRET model was detected using a fluorescence spectrophotometer. The prepared polymer was compounded with the gold nanorods, and fluorescence changes in the prepared composite were analyzed by fluorescence spectroscopy.
A QCM-D system (Q-Sense, Biolin Scientific, Sweden) was used to detect the micromorphological changes of the polymer as a function of pH. A gold-chip (QSX 301) was selected as the detection substrate. The PLH-b-PEG-b-PCL-LA polymer was dissolved in DMSO and fully incubated (24 h) with the chip to modify the polymer on the chip. The disulfide bonds in the polymer can specifically adsorb to gold, and the polymer can be modified on the gold-chip. PBS buffer solutions at pH 5.5 and pH 7.4 were used as mobile phases, respectively, to collect elastic modulus data at different areas of the chip and analyze the stretching state of the polymer in response to pH changes.
As shown in Scheme 1, the synthesis of the polymer involves three steps. First, histidine-N-carboxy anhydride (HisNCA) was prepared by the thionyl chloride dehydration of Nα-Cbz-Nim-DNP-L-histidine. Next, Fmoc-PEG-OH was used for the ring-opening polymerization of ε-caprolactone, and lipoic acid was then used to modify the PCL end. Finally, the Fmoc-deprotected amino terminus was used for the ring-opening polymerization of HisNCA to synthesize the polyhistidine chain segments, which were reduced by mercaptoethanol (RSH) to expose the imidazole groups. The 1H NMR spectra (Figs. S1 and S2 in Supporting information) confirmed the formation of the desired products.

|
Download:
|
| Scheme 1. Synthesis route of PLH-b-PEG-b-PCL-LA polymer. | |
Through transmission electron microscopy (TEM) and dynamic light scattering (DLS) analyses, the main block copolymer of the FRET model and its self-assembly ability were verified. Block copolymers with different block ratios were prepared by adjusting the polyethylene glycol (PEG 4000 Da) and polycaprolactone (4000 Da) quantities; these were used to prepare nanoparticles by dialysis (Fig. 2). As shown in Figs. 2A and B, Fmoc-PEG-b-PCL could be self-assembled into micelles. Furthermore, with modified with histidine units, the self-assembled nanoparticles were not uniform, adopting popcorn-like nanostructures (Figs. 2C and D). Diameters of PLH-b-PEG-b-PCL-LA polymers nanoparticles in different pH (pH 7.4 and 5.5) were test by dynamic light scattering analysis, and the results were 145.5 ± 2.31 nm and 134.5 ±1.65 nm (Fig. S3 in Supporting information). The zeta potential of the nanoparticles in different pH values were –18.633 ± 0.874 mV (pH 7.4) and –14.73 ± 0.058 mV (pH 5.5), respectively.

|
Download:
|
| Fig. 2. TEM photographs of Fmoc-PEG-b-PCL with low (A) and high (B) magnifications. Scale bars were 200 nm and 100 nm, respectively. TEM images of PLH-b-PEG-b-PCL-LA with low (C) and high (D) magnifications. Scale bars were 500 nm and 200 nm, respectively. | |
Spectral matching of the donor and receiver is a crucial element for implementing FRET. In the current model, the near-infrared-fluorescent dye Cy7 with an excitation wavelength close to 808 nm was selected as the receiver fragment. Gold nanorods were used to adjust the intensity as well as to control fluorescence switching. Morphological and spectral analyses performed for the prepared gold nanorods were consistent with the expected donor-receiver configuration of the FRET model (Figs. 3A and B).
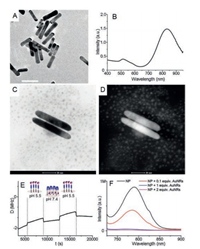
|
Download:
|
| Fig. 3. Characterization and analysis of FRET model. (A) TEM images of gold nanorods. Scale bar: 50 nm. (B) Absorbance spectrum of gold nanorods. (C) TEM observations under the bright field of the FRET model. Scale bar: 20 nm. (D) The image of complexes under the dark field. Scale bar: 20 nm. (E) Structure and state of the polymer on a gold chip detected by quartz crystal microbalance. (F) Fluorescence spectral analysis of the FRET system formed by the compounding of Cy7-labeled PLH-b-PEG-b-PCL-LA and Au NRs. One and two equivalent weights of gold nanorods were added in the physical mixture groups (blue and magenta lines, respectively). | |
A DMSO solution of PLH-PEG-b-PCL-LA was compounded with gold nanorods to complete the assembly of FRET model. Neither bright nor dark-field TEM observations could confirm the presence of polymer on the surface of the gold nanorods (Figs. 3C and D). However, the adsorption and allosteric processes between the polymer and gold substrates could be investigated through detection by quartz crystal microbalance. The energy dissipation (D) of the sensor's oscillation reveals the copolymer film's softness (viscoelasticity). The change in viscoelasticity indicates the folding or curling of the polymer. The polymer was observed to adsorb on the gold chips and effectively respond to the changes in the pH of the mobile phase (Fig. 3E).
The fluorescence signal of the Cy7 unit can be detected by fluorescence spectrophotometry, which confirms a weakening of the dynamic FRET effect in the fluorescence spectrum. The FRET composite model was prepared by the dialysis of a polymer (2 mg/mL) and gold nanorod (50 ng/mL) mixture. In Fig. 3F, the Cy7 fluorescence signal in the gold-complexed model (red line) is compared with the Cy7 signal from the labeled pH-sensitive polymer alone (black line), with physical mixtures of the group as controls (blue and magenta lines). The FRET model quenches the Cy7 signal, but the reduction in signal intensity is not as strong as those in the physical mixture groups. Whether these results will have a negative impact on the low-background imaging of tumors remains to be further verified.
We exploited the self-assembly properties of a triblock copolymer to construct a FRET model, modifying the two ends of the polymer with a fluorescent donor moiety and gold nanorod receptor having matched emission and absorption spectra. The FRET effect was dynamically realized during the self-assembly and folding process, which allowed control of the fluorescence intensity. It provided a potential model for designing and preparing integrated diagnosis and treatment polymers with low background interference. Block copolymers have flexible compositions and can be endowed with new properties by changing the composition of the polymer chain segments. In addition, the folding self-assembly of block copolymers can dynamically change the distance between the two ends of the molecule, providing structural support for the construction of functional materials with tailored physical properties. Doping and coordinating functional metals into organic polymers will provide a broader scheme for the research and development of intelligent materials for medical applications, provide new ideas for the innovative treatment of diseases, and significantly enhance cross-disciplinary development.
Declaration of competing interestWe declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled "Construction of triblock copolymer–gold nanorod composites for fluorescence resonance energy transfer via pH-sensitive allosteric".
AcknowledgmentsWe sincerely acknowledge the funding and generous support of Zhejiang Province Natural Science Foundation (Nos. LY17C100003, Y17E030032), Key Research Project of Traditional Chinese Medicine of Zhejiang Province of China (No. 2019ZZ015), and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2018KY131).
Appendix A. Supplementary dataSupplementary material related to this article can be found, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2020.06.039.
| [1] |
S. Thakkar, D. Sharma, K. Kalia, R.K. Tekade, Acta Biomater. 101 (2020) 43-68. DOI:10.1016/j.actbio.2019.09.009 |
| [2] |
T. Sim, C. Lim, N.H. Hoang, K.T. Oh, J. Pharm. Investig. 47 (2017) 383-394. DOI:10.1007/s40005-017-0349-1 |
| [3] |
Z. Wang, X. Deng, J. Ding, et al., Int. J. Pharm. 535 (2018) 253-260. DOI:10.1016/j.ijpharm.2017.11.003 |
| [4] |
Y. Song, D. Li, J. He, M. Zhang, P. Ni, Chin. Chem. Lett. 30 (2019) 2027-2031. DOI:10.1016/j.cclet.2019.04.052 |
| [5] |
H. Tang, W. Zhao, J. Yu, Y. Li, C. Zhao, Molecules 24 (2019) 4. |
| [6] |
R. Liu, B. He, D. Li, et al., Polymer 53 (2012) 1473-1482. DOI:10.1016/j.polymer.2012.02.013 |
| [7] |
X. Zhang, D. Chen, S. Ba, et al., Colloids Surf. B 140 (2016) 176-184. DOI:10.1016/j.colsurfb.2015.12.032 |
| [8] |
M. Gulfam, F.F. Sahle, T.L. Lowe, Drug Discov. Today 24 (2019) 129-147. DOI:10.1016/j.drudis.2018.09.019 |
| [9] |
D.A. Tomalia, B. Klajnert-Maculewicz, K.A.M. Johnson, et al., Prog. Polym. Sci. 90 (2019) 35-117. DOI:10.1016/j.progpolymsci.2018.09.004 |
| [10] |
W.R. Algar, N. Hildebrandt, S.S. Vogel, I.L. Medintz, Nat. Methods 16 (2019) 815-829. DOI:10.1038/s41592-019-0530-8 |
| [11] |
S. Peng, R. Sun, W. Wang, C. Chen, Chin. Chem. Lett. 30 (2019) 1503-1508. DOI:10.1016/j.cclet.2019.03.033 |
| [12] |
Y. Li, Y. Ban, R. Wang, et al., Chin. Chem. Lett. 31 (2020) 443-446. DOI:10.1016/j.cclet.2019.07.047 |
| [13] |
X. Guo, L. Wang, K. Duval, et al., Adv. Mater. 30 (2018) 1705436. DOI:10.1002/adma.201705436 |
| [14] |
X. Guo, X. Wei, Z. Chen, et al., Prog. Mater. Sci. 107 (2020) 100599. DOI:10.1016/j.pmatsci.2019.100599 |
| [15] |
C. Gong, S. Shi, P. Dong, et al., Int. J. Pharm. 365 (2009) 89-99. DOI:10.1016/j.ijpharm.2008.08.027 |
| [16] |
C. Hu, Z. Chen, S. Wu, et al., Chin. Chem. Lett. 28 (2017) 1905-1909. DOI:10.1016/j.cclet.2017.07.020 |
| [17] |
N. Asadi, N. Annabi, E. Mostafavi, et al., Artif. Cell. Nanomed. B 46 (2018) 938-945. DOI:10.1080/21691401.2018.1439839 |
| [18] |
D.T. Nguyen, V.G. Phan, D.S. Lee, T. Thambi, Polym.Degrad.Stab. 162 (2019) 36-46. DOI:10.1016/j.polymdegradstab.2019.02.013 |
| [19] |
J.V. Brandt, R.D. Piazza, C.C. dos Santos, et al., Colloids Surf. B 177 (2019) 228-234. DOI:10.1016/j.colsurfb.2019.02.008 |
| [20] |
R. Liu, D. Li, B. He, et al., J. Control. Release 152 (2011) 49-56. DOI:10.1016/j.jconrel.2011.02.031 |
| [21] |
Z. Yang, Y. Li, J. Gao, et al., Colloids Surf. B 153 (2017) 111-122. DOI:10.1016/j.colsurfb.2017.02.016 |
| [22] |
S. Shi, Y. Wang, J. Yu, et al., RSC Adv. 5 (2015) 22076-22079. DOI:10.1039/C4RA16012F |
 2020, Vol. 31
2020, Vol. 31 

