Amyloid-β (Aβ) aggregation into fibrils is a pathological hallmark of Alzheimer's disease (AD) [1]. The major isoforms of Aβ are Aβ40 and Aβ42, which differ only in the two extra Cterminal residues. Compared with Aβ42, Aβ40 has higher solubility and lower aggregation propensity, which makes it the preferred peptide for in vitro kinetic analysis [2]. According to previous reports, a variety of materials such as nanoparticles [3, 4], antibodies [5], peptides [6], and organic compounds [7], are interfere with the amyloid aggregation process. Compared with other aggregation interfering agents, the advantages of nanoparticles mainly focus on their high surface ratio and easy functionalization. In addition, nanoparticles can also provide the possibility to pass blood-brain barrier (BBB) [8, 9]. Especially, nanocomposites combine many advantages of different nanoparticles such as better photocatalytic, and optical properties [10, 11]. They have gradually attracted increasing interests in the field of biomedicine. However, only few nanocomposites (graphene oxide-iron oxide; graphene oxide/gold) have been reported to show inhibitory or accelerative effects on amyloid aggregation [12, 13].
As a typical two-dimensional (2D) nanomaterial, molybdenum sulfide nanosheet (MoS2) has attracted wide attention in the field of electronics, biochemistry, and clinical medicine [14]. Compared with MoS2, gold nanoparticle-decorated molybdenum sulfide (AuNP-MoS2) nanocomposites showed superior photocatalytic, electrochemical, and biocompatibility properties than that of MoS2 [15]. Therefore, it was more suitable for biomedical applications. In previous studies, Wang et al. have showed that MoS2 was a promising 2D nanomaterial for modulating amyloid aggregation [16]. Yang et al. showed that MoS2-AuNR nanocomposites could enhance the ability of disrupting Aβ fibrils under NIR irradiation [17]. However, the mechanism and influence of AuNP-MoS2 nanocomposites on amyloid aggregation still remained to be explored. In addition, the concentration effect of nanomaterials on fibrillation was controversial. Ramshini et al. found low concentrations of Ag-NPs could inhibit the aggregation of HEWL, while high concentrations of Ag-NPs could accelerate the aggregation of HEWL [18]. However, Radic et al. used molecular simulation technology to speculate that low NP/protein ratio promoted protein aggregation, while high NP/protein ratio displayed an inhibitive effect [19]. Therefore, the concentration of nanomaterials was critical especially.
In this work, the effect of different concentrations of AuNP-MoS2 nanocomposites on Aβ40 aggregation was studied using atomic force microscopy (AFM), thioflavin T (ThT) fluorescence spectra and circular dichroism (CD) spectra. The results showed that low concentration of AuNP-MoS2 nanocomposites accelerated Aβ40 fibrils aggregation and high concentration of AuNP-MoS2 nanocomposites inhibited Aβ40 aggregation process. The contradictory effect on Aβ40 aggregation may be caused by the interaction between AuNP-MoS2 nanocomposites and Aβ40 peptides, and the distinctive adsorption efficiency of AuNP-MoS2 nanocomposites, which were certified by ultraviolet-visible (UV–vis) absorption spectra and tyrosine intrinsic fluorescence spectra. This work could provide new ideas for investigating the interactions between nanocomposites and amyloid proteins.
AuNP-MoS2 nanocomposites were first prepared by hydrothermal synthesis method [20]. The synthesized AuNP-MoS2 nanocomposites were then characterized. As shown in Fig. S1A (Supporting information), the absorption peak of MoS2 solution was 304 nm. (curve b). After adding HAuCl4 into the MoS2 solution, the absorption peak of the HAuCl4 at 290 nm (curve a) decreased obviously. At the same time, a new absorption peak at around 537 nm emerged, which suggested the consumption of Au3+ and the formation of AuNPs (curve c) [21]. XRD was also used to characterize the structure of AuNP-MoS2 nanocomposites. As shown in Fig. S1B (Supporting information), strong diffraction peaks emerged at 38.2, 44.6, 64.7, 77.7, and 82.0, which could be assigned to the (111, 200, 220, 311, and 222) planes of Au respectively (JCPDS No. 04-0784) [22]. TEM was also used to characterize AuNP-MoS2 nanocomposites. As shown in Fig. S1C (Supporting information), the AuNPs around 15 nm were modified on MoS2 nanosheets. The synthesis of AuNP-MoS2 nanocomposites was also investigated by scanning transmission electron microscope-energy dispersive X-ray spectroscopy (STEM-EDX) maps. As shown in Fig. S1D (Supporting information), there were three elements from the signals, Mo (image b), Au (image c), and S (image d). The above results implied the formation of AuNP-MoS2 nanocomposites.
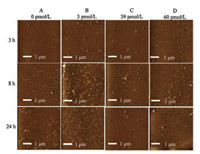
Given that AFM has a comparative advantage in the imaging of biological samples [23-25], the influence of AuNP-MoS2 nanocomposites on Aβ40 aggregation were evaluated by AFM. Fig. 1 showed the AFM images of Aβ40 peptides with different concentrations of AuNP-MoS2 nanocomposites from different time points. As shown in Fig. 1A, the Aβ40 fibrils gradually formed until 24 h as the AuNP-MoS2 nanocomposites were absent. As shown in Fig. 1D, short Aβ40 fibrils could be observed when the incubation time was 3 h. However, without the presence of AuNP-MoS2 nanocomposites, the Aβ40 peptides were still in monomeric or oligomeric status after 3 h of incubation (Fig. 1A). The results showed that AuNP-MoS2 nanocomposites could shorten the nucleation stage of Aβ40 peptides. Furthermore, the number and length of Aβ40 fibrils also increased after Aβ40 peptides incubated with 3 pmol/L AuNP-MoS2 nanocomposites for 8 h and 24 h, which showed the aggregation process was accelerated. However, the number of Aβ40 fibrils decreased slightly after 8 h or 24 h as 39 pmol/L or 60 pmol/L AuNP-MoS2 nanocomposites was present, which showed the aggregation process was inhibited at elongation stage [26].
|
Download:
|
| Fig. 1. AFM images of Aβ40 peptides with various concentrations of AuNP-MoS2 nanocomposites. The concentrations of AuNP-MoS2 nanocomposites were 0 pmol/L (A), 3 pmol/L (B), 39 pmol/L (C) and 60 pmol/L (D), respectively. | |
The effect of AuNP-MoS2 nanocomposites on Aβ40 aggregation was also evaluated by ThT fluorescence spectra [25, 27]. According to the previous research [17], the aggregation of Aβ40 is a two-step reaction. The initial lag period reflected the thermodynamic barrier to form a nucleation "seed", then a rapid fibril propagation and aggregation stage were followed. As shown in Fig. 2A, the addition of AuNP-MoS2 nanocomposites significantly decreased the lag time of Aβ40, implying that the AuNP-MoS2 nanocomposites enhanced the nucleus formation. Interestingly, 3 pmol/L AuNP-MoS2 nanocomposites increased ThT fluorescence intensity of Aβ40 by about 25%, while 39 pmol/L and 60 pmol/L AuNP-MoS2 nanocomposites decreased ThT fluorescence intensity of Aβ40 by almost 30% and 60%, respectively. It indicated that the Aβ40 aggregation process was inhibited. The results of ThT fluorescence were consistent with that of AFM imaging. To further study the effects of AuNP-MoS2 nanocomposites on Aβ40 fibrillation, different concentrations of AuNP-MoS2 nanocomposites (3, 39 and 60 pmol/L) were added to Aβ40 solution at nucleation stage. As shown in Fig. 2B, the Aβ40 peptides were incubated for 1 h to grow to oligomers. Then different concentrations of AuNP-MoS2 nanocomposites were incubated with oligomers solution for 23 h afterwards. The results of kinetic curves showed that there was no significant difference with or without AuNP-MoS2 nanocomposites (Fig. 2B). The results showed that the interaction between AuNP-MoS2 nanocomposites and Aβ40 peptides was at monomeric status. In addition, the effect of MoS2 or AuNPs on Aβ40 aggregation was also discussed. As shown in Fig. S2A (Supporting information), low or high concentration of MoS2 only showed inhibition effect on Aβ40 aggregation. The concentration of MoS2 was consistent with MoS2 in AuNP-MoS2 nanocomposites here, i.e., 0.9 nmol/L MoS2 was consistent with MoS2 in 3 pmol/L AuNP-MoS2 nanocomposites, and 12 nmol/L MoS2 was consistent with MoS2 in 39 pmol/L AuNP-MoS2 nanocomposites. Fig. S2B (Supporting information) showed 3 pmol/L AuNPs had no effect on Aβ40 aggregation, while 39 pmol/L AuNPs showed inhibition effect on Aβ40 aggregation. The results were consistent with the previous researches [16, 28]. Comparing with MoS2 and AuNPs, AuNP-MoS2 nanocomposites showed concentration dependent roles for Aβ40 aggregation.

|
Download:
|
| Fig. 2. ThT fluorescence spectra and CD spectra of Aβ40 peptides with different concentrations of AuNP-MoS2 nanocomposites. The concentrations of AuNP-MoS2 nanocomposites were 0, 3, 39, and 60 pmol/L, respectively. (A) ThT fluorescence spectra of Aβ40 peptides with different concentrations of AuNP-MoS2 nanocomposites. The addition time was 0 h. (B) ThT fluorescence spectra of Aβ40 peptides with different concentrations of AuNP-MoS2 nanocomposites. The addition time was 1 h. (C) CD spectra of Aβ40 peptides with different concentrations of AuNP-MoS2 nanocomposites. | |
CD spectra were also used to investigate the transition of Aβ40 peptides structure with AuNP-MoS2 (Fig. 2C). After Aβ40 peptides were incubated for 24 h, CD spectrum of Aβ40 fibrils showed a negative valley at around 218 nm, which indicated the existence of β-sheet structure [29]. In the presence of AuNP-MoS2 nanocomposites, different changes appeared in the CD spectra after Aβ40 peptides were incubated for 24 h. When the concentration of AuNP-MoS2 nanocomposites was 3 pmol/L, the intensity of negative peak at 218 nm increased, indicating the increase in content of Aβ40 peptides β-sheet. However, when the concentration of AuNP-MoS2 nanocomposites was 39 or 60 pmol/L, the negative peak at 218 nm became weaker, indicating the decrease content of Aβ40 peptides β-sheet. The results showed low concentration of AuNP-MoS2 nanocomposites increased the content of Aβ40 peptides β-sheet, while high concentration of AuNP-MoS2 nanocomposites decreased the content of Aβ40 peptides β-sheet. The CD experiment also supported the results of AFM and ThT fluorescence experiments.
Next, the influence of AuNP-MoS2 nanocomposites on preformed Aβ40 fibrils was evaluated by AFM, and ThT fluorescence spectra. Fig. S3 (Supporting information) showed the AFM images of Aβ40 fibrils in the presence of different concentrations of AuNP-MoS2 nanocomposites from different time points. The results showed that the Aβ40 fibrils dissociated gradually with the incubation of 60 pmol/L AuNP-MoS2 nanocomposites, implying that AuNP-MoS2 nanocomposites could affect the properties of preformed Aβ40 fibrils. In addition, the ThT experiment also verified the results of AFM experiments. As shown in Fig. S4 (Supporting information), as 60 pmol/L of AuNP-MoS2 nanocomposites was present, the ThT fluorescence intensity of Aβ40 fibrils decreased gradually with increasing incubation time, implying the depolymerization of Aβ fibrils. It could be due to fibril sedimentation as described in the previous literature [28]. In addition, 30 pmol/L AuNP-MoS2 nanocomposites were also used to explore the depolymerization of AuNP-MoS2 nanocomposites on preformed Aβ40 fibrils (Fig. S5 in Supporting information). It showed that the effect of 30 pmol/L AuNP-MoS2 nanocomposites was not obvious than that of 60 pmol/L AuNP-MoS2 nanocomposites. The results showed that high concentration of AuNP-MoS2 nanocomposites may depolymerize the Aβ40 fibrils.
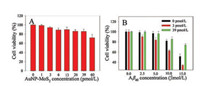
Taken the SH-SY5Y cell as a model, the effects of AuNP-MoS2 nanocomposites on Aβ40 peptides mediated cytotoxicity of cells was then studied [30]. As shown in Fig. 3A, the cell viability was higher than 80% after the cell was treated with concentration of < 39 pmol/L AuNP-MoS2 for 48 h, implying the low cytotoxicity of AuNP-MoS2. The cytotoxicity of Aβ40 peptides with different concentrations of AuNP-MoS2 nanocomposites was then investigated. As shown in Fig. 3B, the cell viability decreased gradually with the treatment of increasing Aβ40 peptide concentration when the AuNP-MoS2 nanocomposites were absent. When the AuNP-MoS2 nanocomposites were present, the effects of different concentrations of AuNP-MoS2 nanocomposites on Aβ40 peptides mediated cytotoxicity of cells were different. Taken 15 μmol/L Aβ40 peptides as an example, when 3 pmol/L AuNP-MoS2 was added to Aβ40 peptides, the cytotoxicity of the peptides increased, and the cell viability decreased from 51.6%–34.1%. On the contrary, the 39 pmol/L AuNP-MoS2 decreased the cytotoxicity of the peptides apparently, and the cell viability increased from 51.6% to about 73.5%. The results showed that different concentrations of AuNP-MoS2 nanocomposites could regulate the neurotoxicity of amyloid peptides. In addition, although 60 pmol/L of AuNP-MoS2 could depolymerize the Aβ40 fibrils (Fig. S4), it is slightly toxic to cells (Fig. 3A). Therefore, it implied the importance of appropriate concentration of AuNP-MoS2 on Aβ40 aggregation.
|
Download:
|
| Fig. 3. (A) Cell viability of SH-SY5Y cells with different concentrations of AuNP-MoS2. The concentrations of AuNP-MoS2 nanocomposites were 0, 1, 3, 6, 13, 26, 39 and 60 pmol/L, respectively. (B) Effects of AuNP-MoS2 nanocomposites with different concentrations (0, 3, 39 pmo/L) on Aβ40-mediated cytotoxicity of SH-SY5Y cells. | |
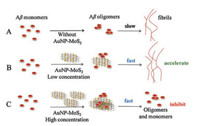
According to the results, it was shown that low concentration of AuNP-MoS2 nanocomposites could not only enhance the nucleus formation of Aβ40 peptides, but also promote Aβ40 fibrils aggregation. However, although high concentration of AuNP-MoS2 nanocomposites could enhance the nucleus formation of Aβ40 peptides, it eventually inhibited Aβ40 aggregation process. The possible mechanism may be explained as shown in Fig. 4. Fig. 4A showed the normal pathway of Aβ40 aggregation without AuNP-MoS2 nanocomposites. In the presence of low concentration of AuNP-MoS2 nanocomposites, it acted as nuclei as demonstrated in the previous works [31], and then Aβ40 peptides aggregated around the AuNP-MoS2 nanocomposites, resulting in the acceleration of the nucleation process (Fig. 4B). With increasing concentration of AuNP-MoS2 nanocomposites, the interaction between AuNP-MoS2 nanocomposites and Aβ40 peptides may limit the structural flexibility of Aβ40 peptides for conformational conversion, resulting in the inhibition of Aβ40 fibrils (Fig. 4C).
|
Download:
|
| Fig. 4. Mechanism diagram of concentration dependent effect of AuNP-MoS2 nanocomposites on Aβ40 aggregation. | |
In the hypothesis, the interaction between AuNP-MoS2 nanocomposites and Aβ40 peptides was mentioned. The UV–vis absorption spectra and tyrosine intrinsic fluorescence spectra were used for investigating the interaction between AuNP-MoS2 nanocomposites and Aβ40 peptides. As shown in Fig. S6 (Supporting information), the absorption peak of AuNP-MoS2 nanocomposites broadened gradually with increasing concentration of Aβ40 peptides, which indicated Aβ40 peptides could trigger the aggregation of AuNP-MoS2 nanocomposites. The interactions between AuNP-MoS2 and Aβ40 peptides could also be proved by tyrosine intrinsic fluorescence. As shown in Fig. S7 (Supporting information), a quenching of the tyrosine fluorescence signal at 315 nm was observed when Aβ40 peptides were incubated with AuNP-MoS2 nanocomposites, which indicated that AuNP-MoS2 could absorb Aβ40 peptides. As a contrast, the tyrosine fluorescence of Aβ40 peptides incubated with AuNPs, or MoS2 was also evaluated (Figs. S8 and S9 in Supporting information). Moreover, the binding constant and the number of binding sites could be got from the double logarithm regression curve (Eq. 1) [32].
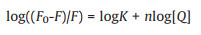
|
(1) |
where F and F0 are the fluorescence intensities with and without AuNP-MoS2 nanocomposites, respectively. K is the binding constant for quencher-protein interaction; n is the number of binding sites of Aβ40 peptides. [Q] is the concentration of AuNP-MoS2 nanocomposites. The values of K and n could be calculated from the slope and intercept by plotting log(F0-F)/F against log[AuNP-MoS2]. The binding constant K and binding sites n of AuNP-MoS2 to Aβ40 peptides were 2.82 × 1015 and 1.36, respectively. The K and n of AuNPs or MoS2 to Aβ40 peptides could be calculated as shown in Table S1 (Supporting information). The results showed that the binding sites of AuNP-MoS2, AuNPs, or MoS2 to Aβ40 peptides were essentially identical. However, the binding constant of AuNP-MoS2 to Aβ40 peptides was much higher than that of AuNPs and MoS2, indicating that AuNP-MoS2 was easier to combine with Aβ40 than AuNPs and MoS2. From the results, it was also speculated that the binding constant for AuNP-MoS2/protein interaction may increase with the increasing surface density of AuNP on MoS2. The contradictory effect of Aβ40 fibrillation was caused by the ratio of peptides adsorbed on AuNP-MoS2 nanocomposites against free peptides in solution. Presumably, higher binding constant may increase the ratio of peptides adsorbed on AuNP-MoS2 nanocomposites, which will reduce the aggregation lag phase of Aβ40, and the concentration border line between the two phenomena.
In summary, we explored the effect of AuNP-MoS2 on Aβ40 aggregation. Compared with AuNPs and MoS2, AuNP-MoS2 nanocomposites played different roles on Aβ40 aggregation. It was concluded that low concentration of AuNP-MoS2 nanocomposites accelerated the nucleation process and elongation process. High concentration of AuNP-MoS2 nanocomposites accelerated the nucleation process, but it eventually inhibited Aβ40 aggregation process. The phenomenon might be caused by the interaction between AuNP-MoS2 nanocomposites and Aβ40 peptides, and the ratio of peptides adsorbed on AuNP-MoS2 nanocomposites against free peptides in solution. The results demonstrated the possibility to regulate amyloid peptide aggregation and depolymerization, which might have potential applications to provide an effective way of treating related diseases.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21375034, 21675047 and 21735002) and Natural Science Foundation for Distinguished Young Scholars of Hunan Province (No. 2016JJ1008).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.052.
| [1] |
Z. Wu, Z. Zhen, J.H. Jiang, et al., Am. Chem. Soc. 131 (2009) 12325-12332. DOI:10.1021/ja9038054 |
| [2] |
A. Espargaró, A. Medina, O.D. Pietro, et al., Sci. Rep. 6 (2016) 23349. DOI:10.1038/srep23349 |
| [3] |
K. Giannousi, O. Antonoglou, C. Dendrinou-Samara, ACS Chem. Neurosci. 10 (2019) 3796-3804. DOI:10.1021/acschemneuro.9b00292 |
| [4] |
D. Lin, R. He, S. Li, et al., ACS Chem. Neurosci. 7 (2016) 1728-1736. DOI:10.1021/acschemneuro.6b00244 |
| [5] |
J. Zhang, J. Xing, L. Wan, et al., Molecules 22 (2017) 334. DOI:10.3390/molecules22030334 |
| [6] |
Z. Zhao, L. Zhu, H. Li, et al., Small 13 (2017) 1602857. DOI:10.1002/smll.201602857 |
| [7] |
H. Zhao, X. Yang, Y. Pan, et al., Chin. Chem. Lett. 31 (2020) 1873-1876. DOI:10.1016/j.cclet.2020.01.042 |
| [8] |
J. Wang, Y. Wei, X. Hu, et al., J. Am. Chem. Soc. 137 (2015) 10576-10584. DOI:10.1021/jacs.5b04894 |
| [9] |
Q. Lin, Z. Li, Q. Yuan, Chin. Chem. Lett. 30 (2019) 1547-1556. DOI:10.1016/j.cclet.2019.06.016 |
| [10] |
H. Liang, P. Hua, Y. Zhou, et al., Chin. Chem. Lett. 30 (2019) 2245-2248. DOI:10.1016/j.cclet.2019.05.046 |
| [11] |
V. Prateek, R. Thakur, Gupta, Chem. Rev. 116 (2016) 4260-4317. DOI:10.1021/acs.chemrev.5b00495 |
| [12] |
I. Ahmad, A. Mozhi, L. Yang, Q, et al., Colloids Surf. B:Biointerfaces 159 (2017) 540-545. DOI:10.1016/j.colsurfb.2017.08.020 |
| [13] |
J. Li, Q. Han, X. Wang, et al., Small 10 (2015) 4386-4394. |
| [14] |
S. Du, Q. Wang, X. Yang, et al., Nanosci. Nanotechno. 17 (2017) 2892-2898. |
| [15] |
X. Liu, P. Liu, Y. Tang, et al., Biosens. Bioelectron. 112 (2018) 193-201. DOI:10.1016/j.bios.2018.04.041 |
| [16] |
J. Wang, L. Liu, D. Ge, et al., Chem. Eur. J. 24 (2018) 3397-3402. DOI:10.1002/chem.201704593 |
| [17] |
X. Wang, Q. Han, X. Liu, et al., Nanoscale 11 (2019) 9185-9193. DOI:10.1039/C9NR01845J |
| [18] |
H. Ramshini, A. Moghaddasi, N. Mollania, et al., J. Iran. Chem. Soc. 16 (2019) 33-44. DOI:10.1007/s13738-018-1478-9 |
| [19] |
S. Radic, T. Davis, P. Ke, et al., RSC Adv. 5 (2015) 105498. |
| [20] |
W. Nie, Q. Wang, X. Yang, et al., Anal. Chim. Acta 993 (2017) 55-62. DOI:10.1016/j.aca.2017.09.015 |
| [21] |
W. Xiao, X. Yan, D. Yu, et al., Mater. Lett. 152 (2015) 128-130. DOI:10.1016/j.matlet.2015.03.118 |
| [22] |
C. Xu, X. Wang, J. Zhu, J. Phys. Chem. C 112 (2018) 10841-10845. |
| [23] |
J. Wang, Y. Cao, Q. Li, et al., Chem. Eur. J. 21 (2015) 9632-9637. DOI:10.1002/chem.201500577 |
| [24] |
Y. Wang, Z. Shen, Z. Guo, et al., Nanoscale 10 (2018) 20007-20012. DOI:10.1039/C8NR06142D |
| [25] |
Y. Zheng, Q. Wang, X. Yang, et al., Anal. Chem. 91 (2019) 1954-1961. DOI:10.1021/acs.analchem.8b04278 |
| [26] |
M. Ma, Y. Wang, N. Gao, et al., Chem. Eur. J. 25 (2019) 11852-11858. DOI:10.1002/chem.201902828 |
| [27] |
M. Nilsson, Methods 34 (2004) 151-160. DOI:10.1016/j.ymeth.2004.03.012 |
| [28] |
Y. Liao, Y. Chang, Y. Yoshiike, et al., Small 8 (2012) 3631-3639. DOI:10.1002/smll.201201068 |
| [29] |
B. Ranjbar, P. Gill, Chem. Biol. Deug. Des. 74 (2009) 101-120. DOI:10.1111/j.1747-0285.2009.00847.x |
| [30] |
D. Ran, X. Wu, J. Zheng, et al., J. Fluoresc. 17 (2007) 721-726. DOI:10.1007/s10895-007-0226-9 |
| [31] |
C. Cabaleiro-Lago, F. Quinlan-Pluck, I. Lynch, et al., ACS Chem. Neurosci. 1 (2010) 279-287. DOI:10.1021/cn900027u |
| [32] |
G. Li, W. Yang, Y. Zhao, et al., Chem. Eur. J. 24 (2018) 13647-13653. DOI:10.1002/chem.201802655 |
 2020, Vol. 31
2020, Vol. 31 

