b School of Pharmacy, Health Science Center, Xi'an Jiaotong University, Xi'an 710061, China
Ulcerative colitis (UC) is a kind of inflammatory bowel disease (IBD) that related to mucosal inflammation and mainly happens in the sites of rectum and colon [1, 2]. Systemic corticosteroids are widely used for the treatment of UC, and among them, budsonide (Bud) is a synthetic glucocorticoid derived from 16-alpha hydroxyprednisolone without the existence of halogen bond on its structure, enabling some characteristics such as superior affinity to hormone receptors, high local potency and ease of removal by primary metabolism in human liver. These advantages make it the most investigated form in IBD and a first-line therapy to cure Crohn's disease [3-6]. Concerning the way of delivery, Bud is traditionally released through rectum, which is inconvenient and unfavorable to the patients. In comparison, oral administration is low cost, flexible and convenient for administration, and also reduces the risk of pain and infection [7-9]. Recently, some Budloaded oral systems are developed and exhibited encouraging preliminary results, while problems such as uncontrollable gastric emptying, fluctuations of gastrointestinal transit and incomplete delivery still to be solved, which are due to undissolved polymer coating on the drug containing tablets [10, 11]. Therefore, developing new oral delivery system is urgently required in both research and application fields [12, 13].
For conventional design strategy, the oral delivery system should be stable in the stomach (pH 1.0–2.5) to prevent drug leakage for a sufficient duration time (8-10 h), and can effectively release drugs in rectum and small intestine [13-17]. To this regard, polymer micelles with amphiphilic core-shell structures are developed as an emerging nano-carrier, their hydrophobic cores can entrap insoluble drugs and the hydrophilic shells make them well-dispersed in the aqueous solution [18-22]. Carboxyl groups are usually introduced to the micelle structures as they can be protonated in low pH environments to enhance the structural stability of the micelles and help them to safely pass through the stomach, and in intestinal tract with elevated pH value the drugs are released due to de-protonation of carboxyl groups and dissociation of the micellar structure [23, 24]. However, it is still challenging for these pH-responsive micelles to accumulate and deliver drugs at the lesion location, which greatly affects the drugabsorbing efficiency and their therapeutic effect.
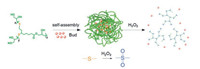
In this work, in order to design a Bud-loaded micellar system to effectively cure UC, a hyperbranched polythioether (HBPTE) with ROS-responsive behavior is designed. Hyperbranched polymers (HBPs) remain at the frontiers of materials research and development due to their one-pot preparation procedures, compact globulelike shapes with abundant terminal functional groups. HBPs can also self-assemble to form micellar structures to entrap hydrophobic drugs and release them in response to external stimuli [25-30]. We follow two principles to design Bud-loaded HBPTE micelles: (1) The micelles should safely pass through the GI tract without structure dissociation or drug leakage; (2) At the inflammatory sites, the micelles can effectively release loaded cargos in response to signals generated from the lesion location. We noticed that the inflammatory lesion has exceptional high level of reactive oxygen species (ROS), thus thioether bond is introduced to be backbone of the designed HBP which is stable in low pH conditions but can be oxidizedto sulphone group by H2O2 (one kind of ROS generated at the inflammatory site), resulting inmuch increased hydrophilicity of the micelles and subsequent release of loaded Bud.
The structure of the designed HBPTE is givenin Fig. 1. To obtain the target polymer, epichlorohydrin is reacted with alpha lipoic acid to replace a hydroxyl group on alpha lipoic acid by an epoxy group. Afterwards, tributyl phosphine is added to break the disulfide bond and induces the ring-opening polymerization (Scheme S1 in Supporting information). The amphiphilic balance of the resulted HBPTE bearing both hydrophobic thioether bonds and hydrophilic hydroxyl groupson thebackbone drives theHBPTEs to self-assemble into micelles that can well disperse in aqueous solution. In the presence of Bud, the self-assembly process can entrap and solubilize the small drug guests. As shown in Fig. S1 (Supporting information), when loaded with Bud, the micellar size increased much, indicating the good encapsulation of Bud molecules within the hydrophobic domain of the compound micelles. When encountering ROS species like hydrogen peroxide, the thioether bonds are oxidized to sulphone groups (Fig. S2 in Supporting information), resulting in much increased hydrophilicity of the HBPTE and subsequent release of the loaded Bud molecules.
|
Download:
|
| Fig. 1. Bud-loaded micelle self-assembled by the synthesized hyperbranched polythioether and its ROS-responsive structural change and Bud release. | |
The effects of H2O2 and pH on cumulative release of Bud-loaded micelles are then investigated. It can be seen from the results shown in Fig. 2a, the micelles show good acid resistance, as for the investigated wide-range of pH value from 1.2–7.4, only 3.3% of the loaded Bud are released from the micelles at maximum. Similar results happened at the samples with H2O2 addition, as the variation of pH value only results in a change of about 5% of the accumulated amount of released Bud. TEM images (Figs. 2b and c) clearly show the structural change of Bud-loaded micelles after H2O2 stimulation, which the originally regular and spherical shape are no longer exist and only precipitates can be seen on the substrate after the treatment of H2O2. This clearly indicates the release of Bud molecules is due to the disruption of the micelles upon the stimulation of H2O2.

|
Download:
|
| Fig. 2. (a) Release of Nano-Bud micelles over time at different H2O2 concentrations and in different pH values. TEM images for Nano-Bud before (b) and after (c) H2O2 treatment. | |
The therapeutic effect of Bud-loaded micelles against dextran sulfate sodium (DSS) induced acute colitis was then investigated. The drug formulations were administrated once per day after 24 h of the induction of colitis. From Fig. 3a it can be seen that the bodyweight of the control group of mice without inducing colitis increased by about 25% of its initial value. In contrast, the group with DSS-induced colitis showed a weight loss of about 20% after 9 days. Moreover, Bud loaded in micelles show better therapeutic effect on alleviating the colitis, as directing feeding the Bud molecules the weight loss of mice is 8%, while this value increase about 3% and 13% for the two groups administrated with low and high concentrations of micelle-loading Bud, respectively. These results clearly suggest the enhanced therapeutic effect of Bud after loading into micelles formed by the synthesized HBPTEs. The Budloaded micelle is named as Nano-Bud in the following context.

|
Download:
|
| Fig. 3. (a) The changes of body weight in each group for 9 d. Effects of Bud and Nano-Bud systems on colonic (b) DAI score and (c) MPO activity in mice with DSS-induced colitis. Data are means ± SEM from a representative experiment (n = 5 biologically independent animals) from two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, analyzed by two-way ANOVA with Tukey's HSD multiple comparison post hoc test. | |
DAI index (disease activity index) and MPO activity are commonly applied to evaluate the extent of colonic inflammation in Ulcerative colitis (UC). The body weight, mental state, stool morphology and bloody stool level (fecal occult blood test) were recorded daily during the modeling period, and the DAI index of each group of mice was also calculated during this time. As shown in Fig. 3b, in the DSS-induced model group, The DAI index was significantly higher than that of the drug-treated groups (P < 0.001) and the normal group (P < 0.0001). At the same time, the group treated using bare Bud showed a gradual increase of the DAI index over time, and began at sixth day this value is notably higher than that of the other two groups treated using Nano-Bud. The lower concentration of the Nano-Bud group was similar to the bare Bud group at the beginning, but the DAI value stopped rising and remained stable on the sixth day. In the higher concentration of Nano-Bud group, the first four days were still significantly higher than the negative control group, but after reaching the maximum on the fourth day, the DAI index decreased rapidly. It proves again that our prepared Nano-Bud can significantly reduce the DAI index and has a good anti-inflammatory effect. Fig. 3c shows the MPO activities caused by modeling, DSS group, bud group and Nano-Bud group. The MPO activity of the colon tissue of the groups treated with Bud or Nano-Bud is much lower compared to the DSS group. Moreover, the Nano-Bud groups show lower MPO value which is positively correlated to their concentrations, indicating the improvement of therapeutic efficiency by loading Bud into the Nano-carriers.
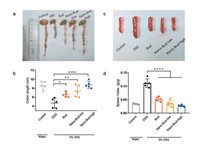
We then investigated the effect of Nano-Bud system on colon length (Figs. 4a and b) and spleen index (Figs. 4c and d) in mice with DSS-induced colitis. As shown in Fig. 4a, DSS treatment resulted in a significant decrease in the length of colon in mice compared to the normal one. After direct bud treatment, the decline of the colon length was reduced, and this reduction is more obvious for the groups treated using Nano-Bud. For that administrated with higher concentration of Nano-Bud, the resulted length of colon is quite close to that of healthy mice. The use of glucocorticoids would induce the atrophy of immune organs and the loss of body weight. Here the influence of DSS on mice spleen was investigated by evaluating the organ index. As is shown in Fig. 4b, the spleen and thymus index of Nano-Bud were significantly improved in comparison with the DSS group (P < 0.01). All data suggested that the Nano-Bud could effectively reduce the side-effects and has an improved therapeutic efficiency on immune organs, which was mainly due to the colon targeting property of Nano-Bud. Thanks to the desirable acid resistance, the Nano-Bud could remain its integration in the stomach and small intestine. Therefore, the side-effects of DSS were significantly reduced because of the reduction of systemic absorption.
|
Download:
|
| Fig. 4. Effects of Nano-Bud system on colon length (a, b) and spleen index (c, d) in mice with DSS-induced colitis. Data are means ± SEM from a representative experiment (n = 5 biologically independent animals) from two independent experiments. **P < 0.01, ***P < 0.001, ****P < 0.0001, analyzed by one-way ANOVA with Tukey's HSD multiple comparison post hoc test. | |
The histopathological features of the mouse colon are shown in Fig. 5. The control group showed the normal colonic structure. In contrast, the DSS group showed necrosis, necrotic mucosal loss, and a strong inflammatory process in the lamina propria, submucosa, and external muscle layers. This situation was alleviated for the Bud treated groups, but necrosis was still observed with a strong inflammatory phenomenon. In the lowconcentration Nano-Bud group, a much normal mucosal structure was observed, and slight chronic inflammation was presented in the muscularis propria. In the high-concentration Nano-Bud group, the image showed an almost completely normal mucosal structure. This indicates that Nano-Bud can significantly treat the inflammation of the lesion compared to direct bud administration and avoid side-effects simultaneously, with a good colon targeting ability. Subsequent colon injury scores also demonstrated these results, as the colon injury score was significantly reduced after the treatment of Nano-Bud, and the higher concentration of Nano-Bud showed a quite close value to that of normal mouse score, indicating that it has a significant protective effect on DSS-induced ulcerative colitis in mice.

|
Download:
|
| Fig. 5. Histology of a representative colon specimen and its colonic damage score in mice with DSS-induced colitis (200 ×). Data are means ± SEM from a representative experiment (n = 5 biologically independent animals) from one independent experiment. **P < 0.01, ***P < 0.001, ****P < 0.0001, analyzed by one-way ANOVA with Tukey's HSD multiple comparison post hoc test. | |
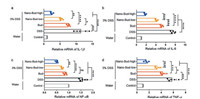
According to the above results, the release of various early inflammatory factors can be observed in the mouse model of ulcerative colitis caused by DSS (Fig. 6). It can be seen that the expression levels of TNF-α, IL-6, IL-1β inflammatory factor mRNA in the intestinal tissue of Bud were significantly decreased (P < 0.05). At the same time, the application of Nano-Bud system has an obviously improved therapeutic effect compared to that of direct Bud administration. After increasing the concentration of Nano-Bud, the expression level is even closer to the control group. NF-κB plays an important role in the inflammatory response via promoting the release of many inflammatory mediators. Compared with the NF-κB in the DSS group, the activation level began to decrease to approaching the control group when only the bud was administrated, which decreased further for the Nano-Bud treated group. This indicates that Nano-Bud system can effectively inhibit the activation of NF-κB induced by DSS.
|
Download:
|
| Fig. 6. Quantitative reverse transcription PCR for the mRNA expression of (a) TNF-α, (b) IL-1β, (c) IL-6 and (d) NF-κB in colon specimen with DSS-induced colitis. Data are means ± SEM from a representative experiment (n = 5 biologically independent animals) from four independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, analyzed by one-way ANOVA with Tukey's HSD multiple comparison post hoc test. | |
In this work, an acid-resistant ROS-responsive micelle selfassembled by hyperbranched polymers of HBPTE and its target delivery of Bud for the treatment of ulcerative colitis is reported. The hydrophobic nature of the HBPTE helps the micelles passes through the low pH locations such as in stomach and safely reaches target sites like colon, while thioether bond could be oxidized to sulphone bond in response to H2O2 to much increase the hydrophilicity of the polymers and the formed micelles, leading to release of loaded Bud molecules. Animal experiments show that the Bud-loaded micelle has an improved therapeutic efficiency on the treatment of UC, preservation of colon length and reduction of spleen index. Quantitative reverse transcription PCR experiments also confirmed that the Bud-loaded micelles can reduce the early inflammatory factors IL-6, TNF-α and IL-1β to improve the progression of DSS-induced ulcerative colitis. In summary, the micelle designed and synthesized in this work has excellent drugdelivery performance and improved therapeutic effect in curing UC.
Declaration of competing interestThere is no declaration of interest in this investigation. The authors declare no competing financial interest.
AcknowledgmentsThis work was supported by the National Nature Science Foundation of China (NSFC, Nos. 51803115, 21636006, 81773686), Nature Science Foundation of Shaanxi Province (No. 2019JQ-528), the Fundamental Research Funds for the Central Universities (Nos. GK201801003, GK201802009, GK201901001), Young Talent Fund of University Association for Science and Technology in Shaanxi, China (No. 20180602), the Program of Introducing Talents of Discipline to Universities (No. B14041).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.039.
| [1] |
K. Wang, K. Dong, Y. Yan, et al., RSC Adv. 5 (2015) 80625-80633. DOI:10.1039/C5RA06884C |
| [2] |
Z.B. Zhao, H.X. Zheng, Y.G. Wei, et al., Chin. Chem. Lett. 18 (2007) 639-642. DOI:10.1016/j.cclet.2007.04.031 |
| [3] |
G.R. Lichtenstein, S.B. Hanauer, W.J. Sandborn, Am. J. Gastroenterol. 104 (2009) 465-483. DOI:10.1038/ajg.2008.168 |
| [4] |
H. Zhou, H.X. Qian, Drug Des. Dev. Ther. 12 (2018) 2601-2609. DOI:10.2147/DDDT.S170676 |
| [5] |
D.S. Bodas, P.P. Ige, Drug Dev. Ind. Pharm. 45 (2019) 1193-1204. DOI:10.1080/03639045.2019.1606823 |
| [6] |
Y. Liu, H. Zhou, Int. J. Mol. Sci. 16 (2015) 2693-2704. DOI:10.3390/ijms16022693 |
| [7] |
J. Zhuang, D.D. Wang, D. Li, et al., Chin. Chem. Lett. 29 (2018) 1815-1818. DOI:10.1016/j.cclet.2018.10.012 |
| [8] |
Y. Zhang, J.J. Chen, G.H. Zhang, et al., React. Funct. Polym. 72 (2012) 359-364. DOI:10.1016/j.reactfunctpolym.2012.03.010 |
| [9] |
Y.C. You, L.Y. Dong, K. Dong, et al., Carbohyd. Polym. 130 (2015) 243-253. DOI:10.1016/j.carbpol.2015.03.075 |
| [10] |
H. Ali, B. Weigmann, M.F. Neurath, et al., J. Control. Release 183 (2014) 167-177. DOI:10.1016/j.jconrel.2014.03.039 |
| [11] |
M. Nolan, L. Tajber, B.F. McDonald, et al., Eur. J. Pharm. Sci. 37 (2009) 593-602. DOI:10.1016/j.ejps.2009.05.007 |
| [12] |
X. Zhu, J. Wu, W. Shan, et al., Angew. Chem. Int. Ed. 55 (2016) 3309-3312. DOI:10.1002/anie.201509183 |
| [13] |
X. Zhu, J. Wu, W. Shan, et al., Adv. Funct. Mater. 26 (2016) 2728-2738. DOI:10.1002/adfm.201505000 |
| [14] |
Y.Q. Yang, L.S. Zheng, X.D. Guo, Y. Qian, L.J. Zhang, Biomacromolecules 12 (2011) 116-122. DOI:10.1021/bm101058w |
| [15] |
Y.N. Xue, Z.Z. Huang, J.T. Zhang, et al., Polymer 50 (2009) 3706-3713. DOI:10.1016/j.polymer.2009.05.033 |
| [16] |
Y.Q. Yang, W.J. Lin, B. Zhao, et al., Langmuir 28 (2012) 8251-8259. DOI:10.1021/la301099q |
| [17] |
L.W. Hsu, Y.C. Ho, E.Y. Chuang, et al., Biomaterials 34 (2013) 784-793. DOI:10.1016/j.biomaterials.2012.09.082 |
| [18] |
C.H. Li, H.M. Li, Q. Wang, et al., J. Control. Release 246 (2017) 133-141. DOI:10.1016/j.jconrel.2016.12.027 |
| [19] |
M.A. Abdelmoneem, M. Mahmoud, A. Zaky, et al., J. Control. Release 287 (2018) 78-93. DOI:10.1016/j.jconrel.2018.08.026 |
| [20] |
Y. Zhou, A.P. Fang, F.Z. Wang, et al., Chin. Chem. Lett. 31 (2020) 494-500. DOI:10.1016/j.cclet.2019.04.048 |
| [21] |
Y.H. Zhang, X.R. Li, Y.X. Zhou, et al., Nanoscale Res. Lett. 5 (2010) 917-925. DOI:10.1007/s11671-010-9583-4 |
| [22] |
K. Yano, Y. Masaoka, M. Kataoka, S. Sakuma, S. Yamashita, J. Pharm. Sci. 99 (2010) 1336-1345. DOI:10.1002/jps.21919 |
| [23] |
Y.Q. Yang, X.D. Guo, W.J. Lin, et al., Soft Matter 8 (2012) 454-464. DOI:10.1039/C1SM06314F |
| [24] |
W.Y. Hua, Z.M. Wu, Q.Q. Yang, et al., Colloids Surf. B:Biointerfaces 183 (2019) 110443-110452. DOI:10.1016/j.colsurfb.2019.110443 |
| [25] |
M. Saraei, R. Sarvari, B. Massoumi, S. Agbolaghic, Polym. Int. 68 (2019) 1795-1803. DOI:10.1002/pi.5890 |
| [26] |
G.X. Cao, G. Li, Q. Yang, et al., Macromol. Rapid Comm. 39 (2018) 1700684. DOI:10.1002/marc.201700684 |
| [27] |
J.B. Yu, H. Chao, G. Li, et al., Macromol. Chem. Phys. 219 (2018) 1800346. DOI:10.1002/macp.201800346 |
| [28] |
H. Chao, G. Li, J.B. Yu, et al., Macromol. Chem. Phys. 220 (2019) 1900004. DOI:10.1002/macp.201900004 |
| [29] |
Y. Ge, P. Li, Y.F. Guan, C.M. Dong, et al., Chin. Chem. Lett. 30 (2019) 1428-1431. DOI:10.1016/j.cclet.2019.03.009 |
| [30] |
Y.Y. Zhuang, H.P. Deng, Y. Su, et al., Biomacromolecules 17 (2019) 2050-2062. |
 2020, Vol. 31
2020, Vol. 31 

