b Hubei Key Laboratory of Wudang Local Chinese Medicine Research, Department of Chemistry, School of Pharmacy, Hubei University of Medicine, Shiyan 442000, China;
c Key Laboratory of Pesticide and Chemical Biology (CCNU), Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, China
The researches on two-dimensional (2D) surface have attracted tremendous attentions in the last decade [1-5]. In fact, the 2D surface exist extensively within many objects in the real world. For example, in biological systems, many of processes such as material transfer by the proteins channels and interactions of viruses with certain cells the strongly are related to interfacial behaviors [6-11]. Inspired by the behaviors in organism, chemist used 2D layered graphene oxide membranes to construct biomimetic gate-induced ion channels [12, 13]. Moreover, the unique optical, electronic, and mechanical properties of 2D interfacial materials served as key components in novel applications for catalysis and optoelectronics [14-18]. The intelligent regulation of 2D surfaces properties can be achieved by imparting the responsive nature of surfaces with vital functionality [19-21]. Hence the functionalization of surface is considered precious and vital. Although some technologies have been described for fabricating the functional 2D surfaces such as electrochemical deposition and calcination, it remains a challenging issue [22-24].
Macrocyclic host-guest systems [25-28], being the conventional focus of supramolecular chemistry and incorporating a novel idea to manage reversible noncovalent interactions between macrocyclic hosts and suitable guests, allow for the construction of various smart surfaces that can adapt to external stimuli, such as self-cleaning surfaces and biomimetic surfaces [29-32]. The emergence of new macrocyclic hosts can enrich the field of supramolecular chemistry due to themacrocyclic hosts such as crown ethers, cyclodextrins, cucurbiturils and calixarenes, have its own special host-guest features [33-45]. Many important reviews have been reported based on macrocyclic host-guest interactions at 2D surfaces in the last few years [46-49]. As one novel type of macrocyclic host, pillararenes are cyclic oligomers through hydroquinone units linked by methylene bridges at the para-positions, accompanying with the characteristics of rigid columnar symmetrical structures and easy modification [50-55]. The specific positive charged and neutral guest molecules can be bound in the electron-rich cavity of pillararenes by non-covalent interactions such as hydrogen bonding, electrostatics, and hydrophobic interactions [56-62]. Due to these merits, the more and more researchers are fascinated by pillararenes, being one of the hot topics in supramolecular chemistry, especially in the area of the functional materials.
This review article aims to outline the very recent and important progresses in the field of hybrid 2D surfaces constructed through pillararenes based host-guest systems (Scheme 1). The review can be divided into five sections according to type of substrates. First, we described the pillararene-based responsive 2D functional materials on Au substrates. Second, we summarized the pillararene-based multilayer films constructed by layer-by-layer on quartz substrates. In Sections 4 and 5, we mainly discussed the self-assembly adsorption behavior of pillararene-based silicon interfaces and the 2D supramolecular composites based pillararenes on graphene substrates. In Section 6, we focused on the applications of hybrid 2D surfaces constructed by pillararenes, especially in the field of smart windows. Finally, it is a summary and outlook on the development of pillararenes based on 2D interfaces.
|
Download:
|
| Scheme 1. The brief outline for tailor and application of hybrid 2D surfaces constructed by pillararenes based host-guest chemistry. | |
2. Pillararenes based Au substrates
Au surfaces possess the good stable physical characteristics and charming chemical properties. Attaching the specific molecules to Au surfaces is of substantial interest for the preparation of advanced nanodevices, leading to various applications [63, 64]. In this section, we discussed pillararenes based 2D functional gold interfaces, including the thermal and chiral responsive behaviors.
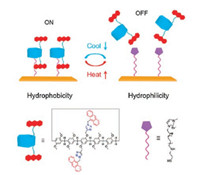
Thermal stimulation is of great interest due to its cheap, clean and easy to obtain characteristics. As shown in Fig. 1, Li and coworkers demonstrated a thermoresponsive switch on Au interface by incorporating the functionalized anthracene pillar[5]arene and imidazolium ion liquid [65]. The host was attached to the Au interface via the self-assembly, leading to fluorescent "ON" with the wettability of surface changing from hydrophilicity to hydrophobicity. Upon the increasing of temperature, the anthracene pillar[5]arene was released because of the disassembly of host-guest, leading to fluorescent "OFF" with the wettability of surface returning to hydrophilicity. Interestingly, the functional interface has high sensitivity and good reversibility toward temperature stimuli through the process of binding and release between host and guest. The above behaviors were confirmed by temperature depending NMR and contact angle (CA) experiments. These studies show that the thermal-responsive host-guest chemistry can be potentially applied in memory storage, drug delivery systems, and sensors.
|
Download:
|
| Fig. 1. Scheme of the temperature-responsive switch constructed by pillar[5]arenebased host–guest interactions. Copied with permission [65]. Copyright 2016, American Chemical Society. | |
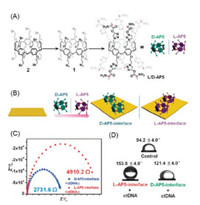
Chirality is one of the fundamental properties of physiological process of life [66-68]. The chiral sensor can be regulated by biological events on the biomimetic surfaces [69]. Thus, the fabrication of chiral interface is conducive to the exploration of chiral phenomena in living systems. Li and his group reported chiral-responsive interfaces introducing D-alanine-pillar[5]arene and L-alanine-pillar[5]arene (D/L-AP5) on the gold interface (Fig. 2) [70]. The D/L-AP5-interfaces were characterized by X-ray photoelectron spectroscopy, atomic force microscope, and electrochemical impedance spectroscopy to confirm the formation of D/L-AP5 based surfaces. More interestingly, the chiral surfaces showed significantly different towards the adsorption amount of calf thymus DNA (ctDNA), which probably explained by the stereotactic hydrogen bond interactions, electrostatic interaction, and chiral selectivity. The method for constructing a chiral responsive interface provides an ideal chiral platform for the study of biological chirality and the development of chiral devices.
|
Download:
|
| Fig. 2. (A) Structures of the L/D-AP5. (B) The schematic of the design and preparation of D/L-AP5-interfaces. (C) Electrochemical impedance spectroscopy of the D/L-AP5-interfaces after immersing them in ctDNA solution. (D) Underwater oil contact angle of the D/L-AP5 interfaces before and after ctDNA adsorption. Copied with permission [70]. Copyright 2019, Royal Society of Chemistry. | |
Similarly, Li and coworkers reported a gold interface modified with chiral phenethylamines (PEA), which can be further assembled with water-soluble pillar[5]arenes (WP5) to achieve highly enantioselective adsorption of lysozyme proteins on the interface [71]. The experimental results showed that the R-PEA-WP5 surface exhibited a stronger preference in lysozyme protein adsorption, which is probably due to the planar chirality of WP5 can be induced by chiral PEA through the host-guest interactions, achieving the chiral amplification in the confined space. On the basis of these findings, the chiral interfaces based on pillararenes offered real promise for constructing biological devices in the adsorption of lysozyme and exploring, and developing the device of chiral recognition.
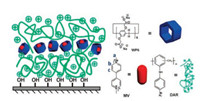
3. Pillararenes based quartz substratesThe approach of layer-by-layer (LbL) assembly has been extensively employed in the design and preparation of multilayer films with explicit components and functions [72]. Multilayer films are normally prepared by continuous adsorption of polyelectrolytes with opposite charges on a solid substrate [73]. Quartz plates are dominantly selected as substrates to prepare multilayers due to the smooth surface and readily available characteristics. In this section, pillararenes were used as building blocks to fabricate multilayer films on quartz substrates, and achieve the uptake and release of guest molecules by host-guest interactions. For example, Zhang and co-workers reported pillar[6]arene-containing multilayer films with artificial binding sites by alternating deposition of diazoresin (DAR) and a complex consisting of anionic pillar[6]arene with methyl viologenanion (MV) (Fig. 3) [74]. The binding site is relatively stabilization even though multilayer films possess the features of high softness and flexibility. The multilayer films can selectively recognize MV guest molecules and showed good reversibility for MV uptake and release, treatment with the mixed solvent of H2O/DMF/ZnCl2 to release MV.
|
Download:
|
| Fig. 3. Schematic representation of multilayer films and the structures of the building blocks for the layer-by-layer assembly. Copied with permission [74]. Copyright 2015, American Chemical Society. | |
Subsequently, Zhang and co-workers discovered that the preparation process of multilayer films no longer required the pre-complexation of the carboxylated pillar[6]arene (WP6) building blocks with MV guest [75]. The pH trigger was introduced to optimize the release system of multilayer films, which can precisely regulate the absorption and release of MV guest molecules. The formation of host-guest stoichiometric complexes with WP6 and MV was studied through UV–vis spectroscopy and illustrated that electrostatic interactions were the main driving force of the complexation. The decrease of pH leads to the disruption of the interaction between WP6 and MV, leading to the release of MV from the multilayer films. The above studies indicate that pillar[6]arene-containing multilayer films have potential application and promising prospect in the area of storage and separation.
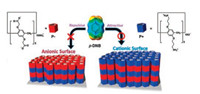
Recently, Ogoshi and co-workers developed an approach for the preparation of pillararene-containing multilayer films with welldefined micropores through LbL assembly [76]. The pillararenebased multilayer films assembled by alternating adsorption of the building block of anionic and cationic pillar[5]arene (P-, P+) (Fig. 4). Compared with the ortho- and meta-dinitrobenzene, the films exhibited preference for adsorption of para-dinitrobenzene (p-DNB) depended on the surface electrostatic potential. Moreover, the capacity of adsorption can be enhanced by increasing the quantity of layers of the films. The pillararene-based multilayer films can uptake and release guest molecules through host-guest interactions. The fabrication of microporous films by the LbL assembly provides a new effective method for the preparation of controlled microporous films.
|
Download:
|
| Fig. 4. Sketch of pillararene-based multilayer films constructed by LbL assembly of anionic pillar[6]arene with cationic pillar[6]arene and size-selective and interface potential-dependent molecular recognition of the films. Copied with permission [76]. Copyright 2015, American Chemical Society. | |
Micro/nanostructured silicon surface can amplify signal output with respect to wettability alterations [77]. Chemists used the silicon wafer as the substrates for the investigation of behaviors of self-assembly, such as the adsorption of proteins, pesticide guests, and enantiomers.
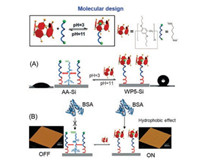
Li and co-workers prepared a pH-driven protein adsorption interface switch on silicon surface [78]. Pillararenes based 2D surfaces were fabricated by three steps. The authors primarily used compound 3-aminopropyltrimethoxysilane to decorate the silicon surface. The guest adipic acid (AA) can further react with 3-aminopropyltrimethoxysilane by classical condensation reaction. Subsequently, the butoxy pillar[5]arene (WP5) host was assembled on the interface via host-guest interactions. The change of contact angle before and after modification displayed that the host-guest chemistry successfully assembled on the interface. Interestingly, the functional surface showed a preference for BSA proteins. The experiment of cyclic CA between base and acid environments exhibited the good reversibility of the interface switch (Fig. 5). Furthermore, the atomic force microscopy confirmed that the interface switch based on WP5 host-guest system can achieve the reversible absorption of BSA through pH regulation. This pillararene-based interface may have the potential applications in the production of new biosensors and immunological tests.
|
Download:
|
| Fig. 5. (A) The WP5 and AA self-assembled host-guest system on a silicon interface and cycling CA between basic and acid environments. (B) Sketch of BSA adsorption interface switch. Copied with permission [78]. Copyright 2016, John Wiley and Sons. | |
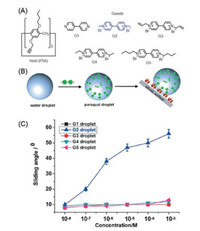
In agricultural field, paraquat is a widely used herbicide [79, 80]. The high toxicity of paraquat brings considerable risks to human health and environment [81, 82]. The adhesion of herbicide droplets on leaf interfaces play an important role in the absorbing herbicides of crops [83, 84]. Li and co-workers reported a functionalized silicon interface to study the dynamic self-assembly adhesion of paraquat on the interface (Fig. 6) [85]. The results of the contact angle hysteresis showed that the paraquat G2 was dynamically self-assembly adhered to the interface, while the other paraquat derivatives G1-G5 slid rapidly from the interface. The quartz crystal microbalance was used to study the binding strength of paraquat droplets on pillar[5]arene-modified functionalized interface. Compared with those of other paraquat derivatives, the adsorption of G2 droplets on the P5A-interface was significantly stronger. The method of dynamic self-assembly adhesion of herbicide droplets on the interface provides a way to improve the efficiency of herbicides.
|
Download:
|
| Fig. 6. (A) Structures of host and guest molecules. (B) Schematic of the dynamic self-assembly of paraquat droplet on the interface. (C) The sliding angle of G1-G5 at different concentrations. Copied with permission [85]. Copyright 2016, John Wiley and Sons. | |
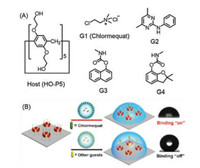
Chlormequat is a widely used plant growth regulator. Li and coworkers reported a pillar[5]arenes-based functional interface to investigate the selective spreading of chlormequat droplets on a hydrophobic interface (Fig. 7) [86]. The selective host-guest recognition between chlormequat and hydroxyl pillar[5]arene (HO-P5) can be used to effective spreading of chlormequat droplets on the hydrophobic interface. The experiment of contact angle measurement demonstrated that the CA value of the interface can be decreased due to the binding of the guests on the HO-P5 interface. It provides a new method for selective spreading of agricultural droplets on the hydrophobic interface and promoting the efficiency of capture for crops.
|
Download:
|
| Fig. 7. Schematic of the chlormequat droplets selected interface and structures of host and guest molecules. Copied with permission [86]. Copyright 2020, American Chemical Society. | |
Subsequently, Li and co-workers fabricated the pillararenebased interfaces which can distinguish histidine enantiomers at the macrolevel [87]. They prepared D-tartaric acid functionalized pillar[5]arene (D-TP5) and discovered that D-TP5 exhibited the chiral discrimination towards histidine enantiomers due to the difference of the binding constant. To develop the chiral device, the chiral interfaces were fabricated by introducing D-TP5 on the silicon interface and characterized by contact angle measurements and attenuated total reflection-Fourier-transform infrared spectroscopy as well as X-ray photoelectron spectroscopy. Interestingly, L-His droplets can be selectively and dynamically adhered to the D-TP5 interface.
5. Pillararenes based graphene substratesBenefiting from excellent electrochemical properties and biocompatibility, graphene and its derivatives are widely used in the field of electrochemistry and biology. We described the pillararene-based supramolecular composites on the surface of graphene and derivatives and their applications in electrochemical sensing and biological imaging.
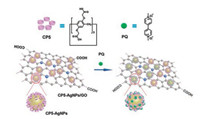
Diao and co-workers used an amphiphilic pillar[5]arene (AP5) to modify the surface of reduced graphene oxide (RGO) for formation of nanocomposite [88]. As-prepared gold nanoparticles (AuNPs) primarily self-assembled onto the surface of RGO-AP5 via amido groups located in AP5 to generate RGO-AP5-AuNPs nanocomposites. Electrochemical results showed that the RGO-AP5 exhibited selective supramolecular recognition and enrichment capability toward guest molecules due to the synergetic action of multifunctional nanocomposites. Similarly, Diao and co-workers reported a nanocomposite constructed by anchoring the carboxylated pillar[5]arene-modified silver nanoparticles (CP5-AgNPs) onto the surface of graphene oxide (GO) through π-π stacking interaction and hydrogen bond between CP5 molecules and GO (Fig. 8) [89]. Notably, the CP5-AgNPs/GO modified glass carbon electrodes (GCE) could be used for the electrochemical detection of paraquat (PQ).
|
Download:
|
| Fig. 8. Schematic of the construction of CP5-AgNPs/GO and sensing paraquat by electrochemical method. Copied with permission [89]. Copyright 2019, Elsevier B.V. | |
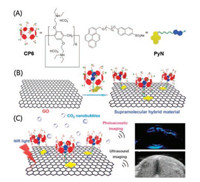
Photoacoustic (PA) imaging combines the merits of ultrasound imaging and optical imaging, which integrates the excellent tissue penetration and high sensitivity. In order to improve the performance of PA imaging, Chen and co-workers reported a supramolecular hybrid material constructed by graphene oxide (GO) and a host-guest complex between tertiary amine functionalized pillar[6]arene (CP6) and the guest (PyN) containing a pyrene tail (Fig. 9) [90]. Driven by π-π stacking between GO and the pyrene, the host-guest complex CP6-PyN is anchored onto the surface of GO to form a hybrid material. Interestingly, upon nearinfrared (NIR) laser irradiation, the bicarbonate counterions on the surface of the hybrid material were decomposed into CO2 nanobubbles due to the NIR light mediated photothermal effect of GO. The experimental results showed that the CO2 nanobubbles act as "molecular boosters" for the significant amplification of ultrasound and PA signal. This supramolecular strategy has developed a distinctive way to construct the imaging-guided therapy.
|
Download:
|
| Fig. 9. (A) Structures of CP6 and PyN. (B) Schematic of supramolecular hybrid material fabricated from GO and the host–guest complex CP6-PyN by hierarchical self-assembly. (C) Schematic of ultrasound and PA signals enhancement under the irradiation of NIR light. Copied with permission [90]. Copyright 2018, the Royal Society of Chemistry. | |
Additionally, Li et al. introduced the competitive host-guest recognition systems onto graphene to fabricate a fluorescent switch, which can be used as a fluorescence probe for paraquat in living cells and mice [91]. Hydrazino-pillar[5]arene-based graphene (HP-G) was easily prepared. The fluorescent switches can be controlled through the competition of guests between paraquat and safranine T indicator. Upon adding the paraquat to the complex formed by HP-G and safranine, it leads to release of fluorescent dye from the cavity of HP-G, along with the enhancement of fluorescence. More importantly, this experiment can be well applied in living cells and mice. This study would be conductive to develop an approach of quantitative living intracellular imaging.
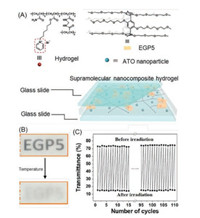
6. Application of the pillararenes based hybrid surfacesThe combination of host-guest systems and substrates endowed the hybrid surfaces with fascinating characteristics. Smart windows are kind of functional materials which can improve the building energy efficiency and indoor comfort through the reversible modulation of the amount of solar radiation entering into the buildings [92, 93]. It is remarkable that thermochromic materials for controlling the transmittance of solar irradiation by thermal stimulation have great potential in the application of smart windows [94, 95]. Wang and co-workers reported a photothermochromic supramolecular nanocomposite hydrogel film based on pyridinium-modified polyacrylamide networks by the combination of antimony-tin oxide (ATO) nanoparticles and ethylene glycol-modified pillar[5]arene (EGP5) (Fig. 10) [96]. Owing to the thermo-responsiveness of EGP5 and plasmonic heating induced by near-infrared (NIR) absorption of ATO, the obtained film exhibits an outstanding photo-thermochromic effect, resulting in good sunlight-induced solar modulation. Meanwhile, the functional film possesses high repeatability and durability because of the reversibility and dynamicity of EGP5-based host–guest interaction. Interestingly, the supramolecular film is used to fabricate smart windows for regulating indoor temperature. The result demonstrates that the smart window obtained by the strategy of supramolecular nanocomposite can preferentially trigger towards sunlight-induced photo-thermochromic effect to decrease an indoor temperature.
|
Download:
|
| Fig. 10. Diagrammatic sketch of sunlight-induced photo-thermochromic film with good cyclic performance for smart window. Copied with permission [96]. Copyright 2018, John Wiley and Sons. | |
Considering the influence of emotion and psychology, the color of light also has a tremendous importance in the applications of smart windows. Wang and co-workers further developed a warm/cool-tone switchable thermochromic hydrogel material for smart windows by orthogonally integrating the properties of ethylene glycol-modified pillar[6]arene (EGP6) and ferrocene (Fc) based on polyacrylamide networks [97]. The hydrogel material (Fcgel·EGP6) exhibited warm/cool tone-switchable property due to the reversible transformation of Fc between orange and green under the stimulation of redox. More importantly, owing to the thermal responsiveness of EGP6, the Fc-gel·EGP6 can regulate the input of solar energy in both warm and cold tones. The host-guest interactions endow the supramolecular materials with the reversibility and dynamicity. In general, by employing the thermochromic effect, the pillararene-based host-guest systems can be used to construct functional materials for smart windows applications. This strategy paves a distinctive way to develop functional materials for smart windows applications.
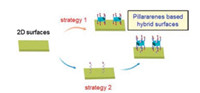
7. Conclusions and outlookIn this feature article, we outlined the fabrication of the functionalized pillararene-based 2D interfaces. It provides an effective way to construct the 2D functional interfaces by introducing the host-guest recognition system of pillararenes into the interface. Pillararenes can bring good properties to the confined surface because of their characteristic of unique structure and easy modification. There are, broadly speaking, two ways of tailoring 2D surfaces: one is to attach specific guests for providing the binding sites, further perfectly reassembling the reasonable pillararenes via host-guest chemistry, the other is to design functional pillararenes directly to be assembled (Fig. 11). However, compared with other mature macrocyclic hosts such as crown ethers, cyclodextrins and calixarenes, continuous efforts are required to investigate the hybrid 2D surface activated by pillararenes. From our point of view, the following three important aspects should be considered in the development of pillararenes on 2D interfaces:
|
Download:
|
| Fig. 11. Schematic strategies for tailoring 2D surfaces with pillararenes based hostguest chemistry. | |
(i) To construct functional interfaces with desired properties, various functional ligands such as polymers can be used to modify pillararenes.
(ii) The construction of 2D interface based on larger pillar[7–10]arenes has rarely explored. The characteristic of cavity would provide the possibility to recognize macromolecules and biomolecules.
(iii) Major efforts in this field have been focused on the construction of 2D pillararene-based interfaces, while relatively less attention to focus on the applications of those of 2D interfaces. The potential applications in materials, biology and environmental science should expand in the future.
It is remarkable that pillararenes can also be modified in artificial nanochannels via similar strategies to construct biomimetic materials [98]. We expect that this feature article can motivate interests and fresh ideas in the field of pillararenes on 2D interface and to develop the various pillararene-based 2D interfaces with novel structures and functions.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the Start-up Funding from South-Central University for Nationalities (No. YZZ19005) and the National Key Research and Development Program of China (No. 2018YFD0200102), the National Natural Science Foundation of China (Nos. 21911530178 and 21772055), the 111 Project (No. B17019) and self-determined research funds of CCNU from the colleges' basic research and operation of MOE, the Open Project of Hubei Key Laboratory of Wudang Local Chinese Medicine Research (Hubei University of Medicine) (No. WDCM2019003).
| [1] |
K.S. Mali, N. Pearce, S. De Feyter, N.R. Champness, Chem. Soc. Rev. 46 (2017) 2520-2542. DOI:10.1039/C7CS00113D |
| [2] |
Q. Fu, X. Bao, Chem. Soc. Rev. 46 (2017) 1842-1874. DOI:10.1039/C6CS00424E |
| [3] |
Z. Cai, B. Liu, X. Zou, H.M. Cheng, Chem. Rev. 118 (2018) 6091-6133. DOI:10.1021/acs.chemrev.7b00536 |
| [4] |
L. Xing, Z. Peng, W. Li, K. Wu, Acc. Chem. Res. 52 (2019) 1048-1058. DOI:10.1021/acs.accounts.9b00002 |
| [5] |
R. Dong, T. Zhang, X. Feng, Chem. Rev. 118 (2018) 6189-6235. DOI:10.1021/acs.chemrev.8b00056 |
| [6] |
X. Zhang, C. Gong, O.U. Akakuru, et al., Chem. Soc. Rev. 48 (2019) 5564-5595. DOI:10.1039/C8CS01003J |
| [7] |
H.X. Zhou, X. Pang, Chem. Rev. 118 (2018) 1691-1741. DOI:10.1021/acs.chemrev.7b00305 |
| [8] |
W. Wang, S. Yu, S. Huang, et al., Chem. Soc. Rev. 48 (2019) 4892-4920. DOI:10.1039/C8CS00402A |
| [9] |
S.L. Liu, Z.G. Wang, H.Y. Xie, et al., Chem. Rev. 120 (2020) 1936-1979. DOI:10.1021/acs.chemrev.9b00692 |
| [10] |
J. Park, B. Andrade, Y. Seo, et al., Chem. Rev. 118 (2018) 1664-1690. DOI:10.1021/acs.chemrev.7b00157 |
| [11] |
A.M. Wen, N.F. Steinmetz, Chem. Soc. Rev. 45 (2016) 4074-4126. DOI:10.1039/C5CS00287G |
| [12] |
L. Shi, C. Mu, T. Gao, et al., J. Am. Chem. Soc. 141 (2019) 8239-8243. |
| [13] |
S.K. Krishnan, E. Singh, P. Singh, M. Meyyappan, H.S. Nalwa, RSC Adv. 9 (2019) 8778-8881. |
| [14] |
J. Gong, X. Bao, Chem. Soc. Rev. 46 (2017) 1770-1771. DOI:10.1039/C7CS90022H |
| [15] |
H. Yin, Z. Tang, Chem. Soc. Rev. 45 (2016) 4873-4891. DOI:10.1039/C6CS00343E |
| [16] |
G. Liu, C. Zhen, Y. Kang, L. Wang, H.M. Cheng, Chem. Soc. Rev. 47 (2018) 6410-6444. DOI:10.1039/C8CS00396C |
| [17] |
J. Cheng, C. Wang, X. Zou, L. Liao, Adv. Opt. Mater. 7 (2019) 1800441. DOI:10.1002/adom.201800441 |
| [18] |
T. Tan, X. Jiang, C. Wang, B. Yao, H. Zhang, Adv. Sci. (2020) 2000058. |
| [19] |
F. Guo, Z. Guo, RSC Adv. 6 (2016) 36623-36641. DOI:10.1039/C6RA04079A |
| [20] |
C. Choi, Y. Lee, K.W. Cho, J.H. Koo, D.H. Kim, Acc. Chem. Res. 52 (2019) 73-81. DOI:10.1021/acs.accounts.8b00491 |
| [21] |
Q. Zhang, J. Zhang, S. Wan, W. Wang, L. Fu, Adv. Funct. Mater. 28 (2018) 1802500. DOI:10.1002/adfm.201802500 |
| [22] |
C. Li, M. Iqbal, J. Lin, et al., Acc. Chem. Res. 51 (2018) 1764-1773. DOI:10.1021/acs.accounts.8b00119 |
| [23] |
Y. Yang, H. Wang, F.Y. Yan, et al., ACS Appl. Mater. Interfaces 7 (2015) 5634-5642. DOI:10.1021/am5088488 |
| [24] |
K.N. Ahmad, S.A. Anuar, W.N.R. Wan Isahak, M.I. Rosli, M.A. Yarmo, ACS Appl. Mater. Interfaces 12 (2020) 7102-7113. DOI:10.1021/acsami.9b18984 |
| [25] |
Y.W. Yang, Y.L. Sun, N. Song, Acc. Chem. Res. 47 (2014) 1950-1960. DOI:10.1021/ar500022f |
| [26] |
G. Yu, K. Jie, F. Huang, Chem. Rev. 115 (2015) 7240-7303. DOI:10.1021/cr5005315 |
| [27] |
F. Huang, L. Isaacs, Acc. Chem. Res. 47 (2014) 1923-1924. DOI:10.1021/ar500212y |
| [28] |
F. Huang, E.V. Anslyn, Chem. Rev. 115 (2015) 6999-7000. DOI:10.1021/acs.chemrev.5b00352 |
| [29] |
M. Zhang, X. Yan, F. Huang, Z. Niu, H.W. Gibson, Acc. Chem. Res. 47 (2014) 3206-3206. DOI:10.1021/ar5003413 |
| [30] |
Y. Sun, J. Ma, D. Tian, H. Li, Chem. Commun. 52 (2016) 4602-4612. DOI:10.1039/C6CC00338A |
| [31] |
L. Tang, Z. Zeng, G. Wang, et al., ACS Appl. Mater. Interfaces 11 (2019) 18865-18875. DOI:10.1021/acsami.9b04948 |
| [32] |
S. Wang, K. Liu, X. Yao, L. Jiang, Chem. Rev. 115 (2015) 8230-8293. DOI:10.1021/cr400083y |
| [33] |
J. Li, D. Yim, W.D. Jang, J. Yoon, Chem. Soc. Rev. 46 (2017) 2437-2458. DOI:10.1039/C6CS00619A |
| [34] |
L. Wang, L. Cheng, G. Li, et al., J. Am. Chem. Soc. 142 (2020) 2051-2058. DOI:10.1021/jacs.9b12164 |
| [35] |
G. Crini, Chem. Rev. 114 (2014) 10940-10975. DOI:10.1021/cr500081p |
| [36] |
Q.D. Hu, G.P. Tang, P.K. Chu, Acc. Chem. Res. 47 (2014) 2017-2025. DOI:10.1021/ar500055s |
| [37] |
R. Kumar, A. Sharma, H. Singh, et al., Chem. Rev. 119 (2019) 9657-9721. DOI:10.1021/acs.chemrev.8b00605 |
| [38] |
X.Y. Lou, Y.W. Yang, Adv. Opt. Mater. 6 (2018) 1800668. DOI:10.1002/adom.201800668 |
| [39] |
Y. Zhou, H. Li, Y.W. Yang, Chin. Chem. Lett. 26 (2015) 825-828. DOI:10.1016/j.cclet.2015.01.038 |
| [40] |
Z. Li, N. Song, Y.W. Yang, Matter 1 (2019) 345-368. DOI:10.1016/j.matt.2019.05.019 |
| [41] |
N. Song, Y.W. Yang, Chem. Soc. Rev. 44 (2015) 3474-3504. DOI:10.1039/C5CS00243E |
| [42] |
J.R. Wu, Y.W. Yang, Chem. Commun. 55 (2019) 1533-1543. DOI:10.1039/C8CC09374A |
| [43] |
K. Wang, Y.W. Yang, Annu. Rep. Prog. Chem. Sect. B:Org. Chem. 109 (2013) 67-87. DOI:10.1039/c3oc90002a |
| [44] |
T. Xiao, W. Zhong, L. Zhou, et al., Chin. Chem. Lett. 30 (2019) 31-36. DOI:10.1016/j.cclet.2018.05.034 |
| [45] |
H. Li, Y.W. Yang, Chin. Chem. Lett. 24 (2013) 545-552. DOI:10.1016/j.cclet.2013.04.014 |
| [46] |
X. Ji, M. Ahmed, L. Long, et al., Chem. Soc. Rev. 48 (2019) 2682-2697. DOI:10.1039/C8CS00955D |
| [47] |
S. Wang, W. Gao, X.Y. Hu, Y.Z. Shen, L. Wang, Chem. Commun. 55 (2019) 4137-4149. DOI:10.1039/C9CC00273A |
| [48] |
S. Dong, B. Zheng, F. Wang, F. Huang, Acc. Chem. Res. 47 (2014) 1982-1994. DOI:10.1021/ar5000456 |
| [49] |
Z. Liu, S.K.M. Nalluri, J.F. Stoddart, Chem. Soc. Rev. 46 (2017) 2459-2478. DOI:10.1039/C7CS00185A |
| [50] |
T. Ogoshi, T.A. Yamagishi, Y. Nakamoto, Chem. Rev. 116 (2016) 7937-8002. DOI:10.1021/acs.chemrev.5b00765 |
| [51] |
M. Xue, Y. Yang, X. Chi, Z. Zhang, F. Huang, Acc. Chem. Res. 45 (2012) 1294-1308. DOI:10.1021/ar2003418 |
| [52] |
D. Cao, H. Meier, Chin. Chem. Lett. 30 (2019) 1758-1766. DOI:10.1016/j.cclet.2019.06.026 |
| [53] |
X. Wu, L. Gao, J. Sun, X.Y. Hu, L. Wang, Chin. Chem. Lett. 27 (2016) 1655-1660. DOI:10.1016/j.cclet.2016.05.004 |
| [54] |
P. Li, Y. Chen, Y. Liu, Chin. Chem. Lett. 30 (2019) 1190-1197. DOI:10.1016/j.cclet.2019.03.035 |
| [55] |
X. Wu, L. Gao, X.Y. Hu, L. Wang, Chem. Rec. 16 (2016) 1216-1227. DOI:10.1002/tcr.201500265 |
| [56] |
T. Xiao, L. Zhou, L. Xu, et al., Chin. Chem. Lett. 30 (2019) 271-276. DOI:10.1016/j.cclet.2018.05.039 |
| [57] |
H. Zhang, Z. Liu, Y. Zhao, Chem. Soc. Rev. 47 (2018) 5491-5528. DOI:10.1039/C8CS00037A |
| [58] |
C. Sathiyajith, R.R. Shaikh, Q. Han, et al., Chem. Commun. 53 (2017) 677-696. DOI:10.1039/C6CC08967D |
| [59] |
Y.F. Li, Z. Li, Q. Lin, Y.W. Yang, Nanoscale 12 (2020) 2180-2200. DOI:10.1039/C9NR09532B |
| [60] |
K. Jie, Y. Zhou, E. Li, F. Huang, Acc. Chem. Res. 51 (2018) 2064-2072. DOI:10.1021/acs.accounts.8b00255 |
| [61] |
N.L. Strutt, H. Zhang, S.T. Schneebeli, J.F. Stoddart, Acc. Chem. Res. 47 (2014) 2631-2642. DOI:10.1021/ar500177d |
| [62] |
N. Song, T. Kakuta, T.A. Yamagishi, Y.W. Yang, T. Ogoshi, Chem 4 (2018) 2029-2053. DOI:10.1016/j.chempr.2018.05.015 |
| [63] |
D. Samanta, A. Sarkar, Chem. Soc. Rev. 40 (2011) 2567-2592. DOI:10.1039/c0cs00056f |
| [64] |
J.C. Love, L.A. Estroff, J.K. Kriebel, R.G. Nuzzo, G.M. Whitesides, Chem. Rev. 105 (2005) 1103-1170. DOI:10.1021/cr0300789 |
| [65] |
J. Bi, X. Zeng, D. Tian, H. Li, Org. Lett. 18 (2016) 1092-1095. DOI:10.1021/acs.orglett.6b00097 |
| [66] |
H.E. Lee, H.Y. Ahn, J. Mun, et al., Nature 556 (2018) 360-365. DOI:10.1038/s41586-018-0034-1 |
| [67] |
M. Liu, L. Zhang, T. Wang, Chem. Rev. 115 (2015) 7304-7397. DOI:10.1021/cr500671p |
| [68] |
J.R. Brandt, F. Salerno, M.J. Fuchter, Nat. Rev. Chem. 1 (2017) 0045. DOI:10.1038/s41570-017-0045 |
| [69] |
P. Kanchanawong, G. Shtengel, A.M. Pasapera, et al., Nature 468 (2010) 580-584. DOI:10.1038/nature09621 |
| [70] |
J. Zhang, Z. Wang, S. Lv, et al., Chem. Commun. 55 (2019) 778-781. DOI:10.1039/C8CC09696A |
| [71] |
H. Yan, J. Ma, F. Zhu, et al., Chem. Asian J. 15 (2020) 1025-1029. DOI:10.1002/asia.201901821 |
| [72] |
T. Akagi, T. Fujiwara, M. Akashi, Angew. Chem. Int. Ed. 51 (2012) 5493-5496. DOI:10.1002/anie.201201586 |
| [73] |
J. Borges, J.F. Mano, Chem. Rev. 114 (2014) 8883-8942. |
| [74] |
B. Yuan, J.F. Xu, C.L. Sun, et al., ACS Appl. Mater. Interfaces 8 (2016) 3679-3685. DOI:10.1021/acsami.5b08854 |
| [75] |
H. Nicolas, B. Yuan, J. Xu, X. Zhang, M. Schonhoff, Polymers 9 (2017) 719. DOI:10.3390/polym9120719 |
| [76] |
T. Ogoshi, S. Takashima, T.A. Yamagishi, J. Am. Chem. Soc. 137 (2015) 10962-10964. DOI:10.1021/jacs.5b07415 |
| [77] |
G. Qing, T. Sun, Angew. Chem. Int. Ed. 53 (2014) 930-932. DOI:10.1002/anie.201306660 |
| [78] |
X. Xiao, G. Nie, X. Zhang, D. Tian, H. Li, Chem. Eur. J. 22 (2016) 941-945. DOI:10.1002/chem.201504076 |
| [79] |
W. Rongchapo, O. Sophiphun, K. Rintramee, S. Prayoonpokarach, J. Wittayakun, Water Sci. Technol. 68 (2013) 863-869. DOI:10.2166/wst.2013.311 |
| [80] |
T.R. Hawkes, Pest Manag. Sci. 70 (2014) 1316-1323. DOI:10.1002/ps.3699 |
| [81] |
Q. Li, X. Peng, H. Yang, H. Wang, Y. Shu, Mol. Pharm. 8 (2011) 2476-2483. DOI:10.1021/mp200395f |
| [82] |
Y. Huang, C. Li, Z. Lin, ACS Appl. Mater. Interfaces 6 (2014) 19766-19773. DOI:10.1021/am504922v |
| [83] |
G. Qing, T. Sun, Angew. Chem.Int. Ed. 53 (2014) 930-932. DOI:10.1002/anie.201306660 |
| [84] |
X. Zhang, J. Li, N. Feng, et al., Org. Biomol. Chem. 12 (2014) 6824-6830. DOI:10.1039/C4OB00792A |
| [85] |
L. Luo, G. Nie, D. Tian, et al., Angew. Chem. Int. Ed. 55 (2016) 12713-12716. |
| [86] |
S. Yu, N. Gilbert, F. Zhu, et al., Langmuir 36 (2020) 1950-1955. DOI:10.1021/acs.langmuir.9b03961 |
| [87] |
J. Ma, H. Yan, J. Quan, et al., ACS Appl. Mater. Interfaces 11 (2018) 1665-1671. |
| [88] |
J. Zhou, M. Chen, J. Xie, G. Diao, ACS Appl. Mater. Interfaces 5 (2013) 11218-11224. |
| [89] |
J. Sun, F. Guo, Q. Shi, et al., J. Electroanal. Chem. 847 (2019) 113221. |
| [90] |
G. Yu, J. Yang, X. Fu, et al., Mater. Horiz. 5 (2018) 429-435. |
| [91] |
X. Mao, T. Liu, J. Bi, et al., Chem. Commun. 52 (2016) 4385-4388. |
| [92] |
F. Bella, G. Leftheriotis, G. Griffini, et al., Adv. Funct.Mater. 26 (2016) 1127-1137. |
| [93] |
H.Y. Lee, Y. Cai, S. Bi, et al., ACS Appl. Mater. Interfaces 9 (2017) 6054-6063. |
| [94] |
H.Y. Lee, Y. Cai, S. Velioglu, et al., Chem. Mater. 29 (2017) 6947-6955. |
| [95] |
Y. Zhou, Y. Cai, X. Hu, Y. Long, J. Mater. Chem. A 2 (2014) 13550-13555. |
| [96] |
Z. Xu, S. Wang, X.Y. Hu, et al., Sol. RRL 2 (2018) 1800204. |
| [97] |
S. Wang, Z. Xu, T. Wang, et al., Nat. Commun. 9 (2018) 1737. |
| [98] |
Y. Sun, F. Zhang, J. Quan, et al., Nat. Commun. 9 (2018) 2617. |
 2020, Vol. 31
2020, Vol. 31 

