b State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
It is known that 2H-pyrrol-2-ones are the unsaturated conjugated derivatives of γ-lactams that are the core structure in numerous bioactive natural products [1]. Additionally, there are many 2H-pyrrol-2-one derivatives showing assorted pharmaco-logical activities such as FPR1 antagonists [2a], antivirals HIV-1 [2b], antitumorals [2c] or anticancer VEGF-R enzyme inhibitors [2d]. Thus, continuous efforts have been given to the preparation of 2H-pyrrol-2-one derivatives [3]. As part of our program for the generation natural product-like compounds for diverse biological evaluations [4], we are interested in the method development and library construction of 2H-pyrrol-2-one derivatives.
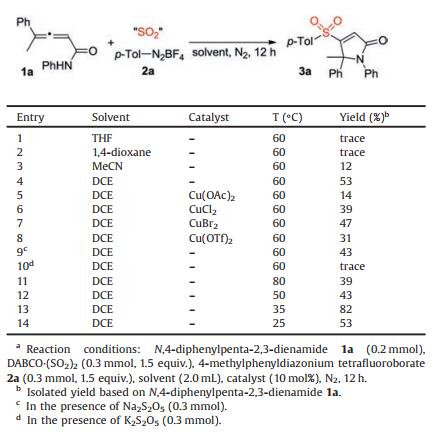
Due to the importance of sulfonyl-containing compounds in pharmaceuticals and agrochemicals [5], we have focused on the synthesis of sulfonyl-containing compounds with the insertion of sulfur dioxide, using DABCO·(SO2)2 and inorganic sulfites as the sulfur dioxide surrogates [6-8]. For instance, we have developed a copper-catalyzed three-component reaction of O-acyl oximes, DABCO·(SO2)2, and 2H-azirines under mild conditions, providing an efficient route for the construction of various tetrasubstituted β-sulfonyl N-unprotected enamines in moderate to good yields with excellent stereoselectivity and regioselectivity [8c]. Synthesis of sulfonyl compounds via a radical process has been demonstrated through the combination of aryldiazonium tetrafluoroborate and DABCO·(SO2)2 [7]. This transformation proceeds mild conditions through a single electron transfer (SET), and arylsulfonyl radical is generated in situ as a key intermediate. Encouraged by these results using sulfur dioxide as the source of sulfonyl group [6-9], we envisioned that formation of sulfonated 2H-pyrrol-2-ones would be feasible by using the strategy of sulfur dioxide inertion. The design is shown in Scheme 1. We conceived that allenoic amides would be the choice for reaction development. After generation of arylsulfonyl radical from aryldiazonium tetrafluoroborate and DABCO·(SO2)2 [7], allenoic amides would react with arylsulfonyl radical. It is expected that the reaction would proceed with excellent chemoselectivity and regioselectivity, thus leading to allylic radical intermediate. Under suitable conditions, the subsequent oxidative single electron transfer would occur giving rise to allylic cation, which would undergo intramolecular nulceophilic attack to provide the corresponding 3-sulfonated 2H-pyrrol-2-ones. Herein, we describe the generation of 3-sulfonated 2H-pyrrol-2-ones through a three-component reaction of allenoic amides, sulfur dioxide, and aryldiazonium tetrafluoroborates under metal-free conditions. This transformation proceeds under mild conditions without the addition of catalysts or additives, giving rise to 3- sulfonated 2H-pyrrol-2-ones in moderate to good yields. Good functional group compatibility is observed. A plausible mechanism is proposed, which is initiated by aryl radicals formed in situ from aryldiazonium tetrafluoroborates and DABCO·(SO2)2. Additionally, excellent chemoselectivity and regioselectivity are presented in this transformation.
|
Download:
|
| Scheme 1. Generation of 3-sulfonated 2H-pyrrol-2-ones. | |
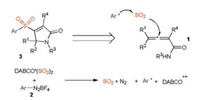
Initially, a model reaction of N, 4-diphenylpenta-2, 3-dienamide 1a, 4-methylphenyldiazonium tetrafluoroborate 2a and DABCO·(SO2)2 was selected and explored. At the beginning, this reaction was performed in THF at 60 ℃ (Table 1, entry 1). However, only a trace amount of product was detected after 12 h. A similar result was observed when the solvent was changed to 1, 4-dioxane (entry2).Toourdelight, thedesiredproduct3-sulfonated2H-pyrrol-2-one 3a was obtained in 12% yield when the reaction occurred in MeCN (entry 3). The yield was dramatically increased to 53% when DCE was used as a replacement (entry 4). Since copper catalyst might assist the single electron transferfrom radicalintermediate to cation, thus several copper salts were added in the reaction system (entries 5–8). However, the results were inferior. Subsequently, other sulfur dioxide surrogates were evaluated. The corresponding product 3a was afforded in 43% yield when sodium metabisulfite was used as the source of sulfur dioxide (entry 9). A trace amount of product was generated when potassium metabisulfite was utilized (entry 10). The reaction temperature was further screened (entries 11–14). It was found that the temperature affected thefinaloutcome obviously. The reaction proceeded efficiently at 35 ℃, giving rise to the desired product 3a in 82% yield (entry 13). Additionally, we examined the reactions by using sodium/potassium metabisulfite as the replacement of DABCO·(SO2)2. However, the yields were low (< 10%).
|
|
Table 1 Initial studies for the reaction of N, 4-diphenylpenta-2, 3-dienamide 1a, 4-methylphenyldiazonium tetrafluoroborate 2a and sulfur dioxide. a |
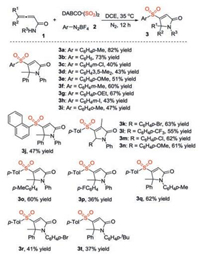
With the optimized conditions in hand, we further explored the substrate scope of this three-component reaction of allenoic amides 1, sulfur dioxide, and aryldiazonium tetrafluoroborates 2 under metal-free conditions. The results are summarized in Scheme 2. A range of allenoic amides 1 and aryldiazonium tetrafluoroborates 2 was examined. It seemed that all reactions proceeded smoothly under the conditions, giving rise to the corresponding 3-sulfonated 2H-pyrrol-2-ones 3 in moderate to good yields. Additionally, various functional groups were compatible. For example, compound 3g was afforded in 67% yield when aryldiazonium tetrafluoroborate with ethoxy group attached on the aromatic ring was employed as the substrate. Reaction of 3-iodophenlydiazonium tetrafluoroborate worked smoothly as well, leading to the desired product 3h in 43% yield. Reactions of other allenoic amides with 4-methylphenyldiazonium tetrafluoroborate 2a and DABCO·(SO2)2 were explored subsequently. As expected, the corresponding 3-sulfonated 2H-pyrrol-2-ones 3 were generated, albeit in low yields in some cases.
|
Download:
|
| Scheme 2. Scope exploration for the reaction of 2, 3-dienamides 1, DABCO·(SO2)2, and aryldiazonium tetrafluoroborates 2. Isolated yields based on allenoic amides 1. | |
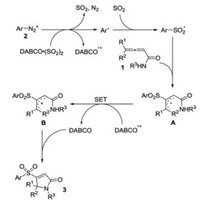
Since the formation of arylsulfonyl radicals by treatment of aryldiazonium tetrafluoroborates and DABCO·(SO2)2 has demonstrated [7], we therefore examined the reaction of N, 4-diphenylpenta-2, 3-dienamide 1a, 4-methylphenyldiazonium tetrafluoroborate 2a and DABCO·(SO2)2 in the presence of 2, 2, 6, 6-tetramethyl-1-piperidinyloxy (TEMPO) under the standard conditions. As expected, the reaction was completely hampered. On the basis of this control experiment and previous reports [7, 8], we therefore proposed a possible mechanism as shown in Scheme 3. We reasoned that initially, the combination of aryldiazonium tetrafluoroborates and DABCO·(SO2)2 would provide aryl radical, sulfur dioxide, nitrogen, and tertiary amine (DABCO) radical cation. Then aryl radical would be trapped by sulfur dioxide, leading to arylsulfonyl radical. The arylsulfonyl radical would attack the central carbon of allenoic amide 1, affording allylic radical intermediate A, which would undergo a single electron transfer (SET) to provide allylic cation B by the assistance of DABCO radical cation. Finally, in the presence of base (DABCO), the amide would act as a nucleophile to attack the allylic cation B, giving rise to the corresponding 3-sulfonated 2H-pyrrol-2-one 3.
|
Download:
|
| Scheme 3. Plausible mechanism. | |
In conclusion, we have developed a facile route to 3-sulfonated 2H-pyrrol-2-ones through a three-component reaction of allenoic amides, sulfur dioxide, and aryldiazonium tetrafluoroborates under metal-free conditions. This transformation proceeds smoothly under mild conditions without the addition of any catalysts or additives, giving rise to 3-sulfonated 2H-pyrrol-2-ones in moderate to good yields. Good functional group compatibility is observed. A plausible mechanism is proposed, which is initiated by aryl radicals formed in situ from aryldiazonium tetrafluoroborates and DABCO·(SO2)2. Additionally, excellent chemoselectivity and regioselectivity are presented in this transformation.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsFinancial support from the National Natural Science Foundation of China (Nos. 21871053 and 21532001) and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No. 2019R01005) is gratefully acknowledged.
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.08.037.
| [1] |
(a) K.C. Nicolaou, L. Shi, M. Lu, et al., Angew. Chem. Int. Ed. 53 (2014) 10970-10974; (b) H. Uchiro, N. Shionozaki, R. Tanaka, et al., Tetrahedron Lett. 54 (2013) 506-511; (c) R. Tanaka, K. Ohishi, N. Takanashi, et al., Org. Lett. 14 (2012) 4886-4889. |
| [2] |
(a) L.N. Kirpotina, I.A. Schepetkin, A.I. Khlebnikov, et al., Biochem. Pharmacol. 142 (2017) 120-132; (b) K. Ma, P. Wang, W. Fu, et al., Bioorg. Med. Chem. Lett. 21 (2011) 6724-6727; (c) C. Zhuang, Z. Miao, L. Zhu, et al., J. Med. Chem. 55 (2012) 9630-9642; (d) C. Peifer, R. Selig, K. Kinkel, et al., J. Med. Chem. 51 (2008) 3814-3824. |
| [3] |
X. del Corte, A. López-Francés, A. Maestro, et al., J. Org. Chem. 85 (2020) 14369-14383. DOI:10.1021/acs.joc.0c00280 |
| [4] |
(a) K.E.O. Ylijoki, J.M. Stryker, Chem. Rev. 113 (2013) 2244-2266; (b) M.E. Garst, B.J. McBride, J.G. Douglass Ⅲ, Tetrahedron Lett. 24 (1983) 1675-1678. |
| [5] |
(a) J.T. Palmer, D. Rasnick, J.L. Klaus, D. Brömme, J. Med. Chem. 38 (1995) 3193-3196; (b) D.C. Meadows, T.B. Mathews, T.W. North, et al., J. Med. Chem. 48 (2005) 4526-4534; (c) D.C. Meadows, J. Gervay-Hague, Med. Res. Rev. 26(2006) 793-814; (d) R. Ettari, E. Nizi, M.E. Di Francesco, et al., J. Med. Chem. 51 (2008) 988-996; (e) R. van der Westhuyzen, E. Strauss, J. Am. Chem. Soc. 132 (2010) 12853-12855. |
| [6] |
(a) G. Qiu, K. Zhou, L. Gao, J. Wu, Org. Chem. Front. 5 (2018) 691-705; (b) K. Hofman, N. Liu, G. Manolikakes, Chem. Eur. J. 24 (2018) 11852-11863; (c) G. Liu, C. Fan, J. Wu, Org. Biomol. Chem. 13 (2015) 1592-1599; (d) G. Qiu, L. Lai, J. Cheng, J. Wu, Chem. Commun. 54 (2018) 10405-10414; (e) G. Qiu, K. Zhou, J. Wu, Chem. Commun. 54 (2018) 12561-12569; (f) A.S. Deeming, E.J. Emmett, C.S. Richards-Taylor, M.C. Willis, Synthesis 46 (2014) 2701-2710; (g) S. Ye, M. Yang, J. Wu, Chem. Commun. 56 (2020) 4145-4155; (h) S. Ye, G. Qiu, J. Wu, Chem. Commun. 55 (2019) 1013-1019; (i) F.S. He, M. Yang, S. Ye, J. Wu, Chin. Chem. Lett. 31 (2020), doi: http://dx.doi.org/10.1016/j.cclet.2020.04.043. |
| [7] |
(a) D. Zheng, Y. An, Z. Li, J. Wu, Angew. Chem. Int. Ed. 53 (2014) 2451-2454; (b) D. Zheng, J. Yu, J. Wu, Angew. Chem. Int. Ed. 55 (2016) 11925-11929; (c) X. Gong, M. Wang, S. Ye, J. Wu, Org. Lett. 21 (2019) 1156-1160; (d) S. Ye, D. Zheng, J. Wu, G. Qiu, Chem. Commun. 55 (2019) 2214-2217; (e) S. Ye, Y. Li, J. Wu, Z. Li, Chem. Commun. 55 (2019) 2489-2492; (f) X. Gong, X. Li, W. Xie, J. Wu, S. Ye, Org. Chem. Front. 6 (2019) 1863-1867; (g) S. Ye, T. Xiang, X. Li, J. Wu, Org. Chem. Front. 6 (2019) 2183-2199; (h) J. Zhang, W. Xie, S. Ye, J. Wu, Org. Chem. Front. 6 (2019) 2254-2259; (i) F.S. He, X. Gong, P. Rojsitthisak, J. Wu, J. Org. Chem. 84 (2019) 13159-13163; (j) S. Ye, X. Li, W. Xie, J. Wu, Asian J. Org. Chem. 8 (2019) 893-898. |
| [8] |
(a) K. Zhou, J.B. Liu, W. Xie, S. Ye, J. Wu, Chem. Commun. 56 (2020) 2554-2557; (b) S. Ye, K. Zhou, P. Rojsitthisak, J. Wu, Org. Chem. Front. 7 (2020) 14-18; (c) J. Zhang, M. Yang, J.B. Liu, F.S. He, J. Wu, Chem. Commun. 56 (2020) 3225-3228; (d) X. Gong, M. Yang, J.B. Liu, F.S. He, X. Fan, J. Wu, Green Chem. 22 (2020) 1906-1910; (e) X. Gong, M. Yang, J.B. Liu, F.S. He, J. Wu, Org. Chem. Front. 7 (2020) 938-943. |
| [9] |
(a) Y. Li, S. Chen, M. Wang, X. Jiang, Angew. Chem. Int. Ed. 59 (2020) 8907-8911; (b) Y. Meng, M. Wang, X. Jiang, Angew. Chem. Int. Ed. 59(2020) 1346-1353; (c) C.M. Huang, J. Li, S.Y. Wang, S.J. Ji, Chin. Chem. Lett. 31 (2020) 1923-1926; (d) M. Wang, J. Zhao, X. Jiang, ChemSusChem 12 (2019) 3064-3068; (e) M. Wang, Q. Fan, X. Jiang, Green Chem. 20 (2018) 5469-5473; (f) L.W. Ye, C. Shua, F. Gagosz, Org. Biomol. Chem. 12 (2014) 1833-1845; (g) Y. Park, S. Chang, Nat. Catal. 2 (2019) 219-227; (h) Q. Xing, C.M. Chan, Y.W. Yeung, W.Y. Yu, J. Am. Chem. Soc. 141 (2019) 3849-3853; (i) B.H. Zhu, C.M. Wang, H.Y. Su, L.W. Ye, Chin. J. Chem. 37 (2019) 58-62; (j) C. Shu, M.Q. Liu, S.S. Wang, L. Li, L.W. Ye, J. Org. Chem. 78 (2013) 3292-3299. |
 2020, Vol. 31
2020, Vol. 31