b State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
Rearrangement manipulations of compounds are widely found in the chemical transformations taking place both in scientific laboratories and nature [1, 2]. In particular, scientists have studied the rearrangement properties of molecules bearing unsaturated bonds, such as carbonyl- and alkynyl-containing compounds, for decades [2]. If applied appropriately, synthetic chemists may synthesize the complex natural products from simple substrates. In fact, reconstruction manipulations possess many benefits over traditional general transformations, such as innate scalability, high atom economy, functional group tolerance and high degree of regioselectivity and stereoselectivity [1, 2].
Pyrone moiety is widely found in the core structure of organic synthetic intermediates, natural products and bioactive compounds [3]. For example, the kojic acid and maltol which have been extensively investigated and numerous transformations have been described in organic synthesis [3-5]. Traditionally, Kojic acid and maltol have been widely used in [5 + 2] cycloaddition reactions, during which seven-membered rings can be formed in a single step [4]. Maltol-type [5 + 2] cycloaddition reactions involve the formation of an oxidopyrylium zwitterion as the reactive intermediate. As shown in Scheme 1a, the main body structure of substrates can also be found in the products in these transformations [5]. Additionally, a bridging oxygen atom can be formed during the process. The related examples of the inter- and intramolecular [5 + 2] cycloaddition reactions occur upon treatment of zwitterionic intermediates with unsaturated carbon-carbon double bonds.

|
Download:
|
| Scheme 1. Pyrone-based transformations. | |
In pyrone-based rearrangement, kojic acids and maltols are commonly used as substrates to generate benzofurans and benzaldehydes. As shown in Scheme 1b, compared with [5 + 2] cycloaddition reactions, the deconstructive reorganization strategies are general description of the bond-breaking reactions of C—O bonds and the construction of benzofurans or benzaldehydes in a single step.
The cascade Claisen rearrangement of the pyrone-containing substrates, such as maltol propargyl ether 1, was reported (Scheme 2) [6]. In this case, the thermal [3, 3]-sigmatropic rearrangement of maltol propargyl ether 1 to the corresponding 1, 5-ene-allene intermediate 4 occurred under the high temperature. And then, the intermediate 4 was subjected to a second Claisen rearrangement to provide the aldehyde 5 that was converted to the key intermediate 6 after the keto-enol tautomerization. The 1, 6-Michael addition of intermediate 6 was achieved by treatment with an alkene 2 and the resulting 7 could be transformed to the intermediate 8 after Friedel-Crafts alkylation. Finally, the generated species 8 underwent intramolecular 1, 5- hydrogen transfer reaction to give the final product. In this transformation, the maltol moiety was taken apart and then reorganized into a benzene ring bearing an aldehyde group.
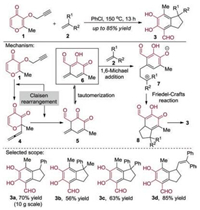
|
Download:
|
| Scheme 2. Cascade Claisen rearrangement of the pyrone-containing substrates: general strategy, mechanism and selected scope of the products. | |
Preparation of polysubstituted salicylaldehydes by this cut-and-sew strategy was described (Scheme 3) [7]. Under the similar reaction conditions, Zhu and co-workers utilized an intramolecular double Claisen rearrangement for the efficient construction of the p-quinone-methide framework 12 which was similar to intermediate 6. Subsequently, the vinyl ether, trimethoxybenzene and benzylthiol could be used as nucleophiles to trap the intermediate 12 leading to the final products.
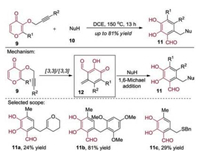
|
Download:
|
| Scheme 3. Preparation of polysubstituted salicylaldehydes by the cut-and-sew strategy: general strategy, mechanism and selected scope of the products. | |
It is well-known that keto alkynes can undergo cyclization to form isomeric products [2a]. This deconstructive reorganization strategy was demonstrated for the synthesis of benzofuran-containing products from the kojic acid- and maltol-derived alkynes (Scheme 4) [8]. In this case, activation of the carboncarbon triple bond with the indium salt generated a π-complex 15, which could undergo the 5-endo-dig cyclization to provide the pyrylium 17. The highly activated intermediate 17 could be hydrolyzed to the stabilized 1, 5-dicarbonyl compound 18, which would then convert to ketol 19 through the intramolecular aldol cyclization. The generated intermediate 19 underwent dehydration and aromatization giving rise to the dihydroxylated benzofuran 14. As the six-carbon synthons, the kojic acid and maltol moieties of the substrates were deconstructed and then reorganized into the hydroxylated benzene rings. At the beginning, kojic acid-derived alkynes with a hydroxyl group were readily converted to the corresponding C5, C6-dihydroxyl benzofurans using In(OTf)3 as the alkynophilic catalyst. Under the same reaction conditions, other reactants could be converted into the corresponding C6-substituted, C5, C6-disubstituted and C4, C5, C6- trisubstituted benzofurans. As a result, four kinds of substituted benzofurans were synthesized with kojic acid-derived alkynes as the starting materials. Encouraged by the positive results described above, other substituted benzofurans could be prepared under the conditions when maltol derivatives were used as the substrates. Likewise, maltol-derived alkynes could also be easily transformed into the C4-substituted and C4, C5-disubstituted benzofurans bearing an unprotected hydroxyl group in good yields.
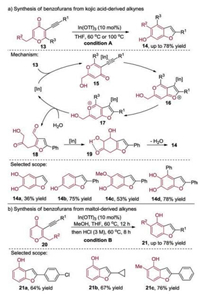
|
Download:
|
| Scheme 4. Substrate scope for the cycloisomerization of kojic acid- and maltolderived alkynes: general strategy, mechanism and selected scope of the products. | |
The hydroxylated benzofuran moiety is widely found in the structure of drug candidates and natural products [9]. This deconstructive reorganization strategy mentioned above would be useful for the synthesis of natural products containing the benzofuran moiety. In the past, the major challenge was how to construct the substituents at different positions of the benzofuran scaffold. Now, this challenge can be solved with this new strategy. Treatment of kojic acid- and maltol-derived alkynes under the general reaction conditions provided the hydroxylated benzofurans, which were subsequently subjected to derivatization with different reagents providing diverse hydroxylated benzofuran-containing natural products. To confirm the practicability of this methodology, various benzofuran-containing natural products, such as Cuspidan B, Alfafuran, Coumestrol and Wittifuran E, were synthesized (Scheme 5) [8].
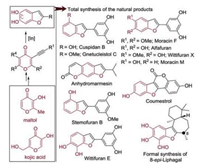
|
Download:
|
| Scheme 5. Total synthesis of benzofuran-containing natural products. | |
In summary, the highlighted reports demonstrate the unique application of deconstructive reorganization strategy to unlock new reactivity in organic synthesis. This strategy is general description of the intramolecular bond-breaking reactions of several old bonds and the construction of several new bonds in one step. With this novel method, the main body structure of kojic acids and maltols, as a five- or six-carbon synthon, was taken apart and then reorganized into a benzene ring bearing an unprotected hydroxyl group, and a series of benzofuran-containing natural products have been constructed. In this strategy, kojic acid and maltol are analogous to the "Transformers" which are characters in Marvel comics. Under the synthetic standpoint, this deconstructive reorganization strategy features high atom economy, innate scalability and functional group tolerance. In the near future, we believe that this unique method will be widely investigated and other novel transformations of kojic acid and maltol will be discovered.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsFinancial support from the National Natural Science Foundation of China (Nos. 21871053 and 21532001) and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No. 2019R01005) is gratefully acknowledged.
| [1] |
(a) Z.M. Chen, X.M. Zhang, Y.Q. Tu, Chem. Soc. Rev. 44 (2015) 5220-5245; (b) J.B. Sweeney, Chem. Soc. Rev. 38 (2009) 1027-1038; (c) R.A. Fernandes, P. Kattanguru, S.P. Gholap, D.A. Chaudhari, Org. Biomol. Chem. 15 (2017) 2672-2710; (d) Z.L. Song, C.A. Fan, Y.Q. Tu, Chem. Rev. 111 (2011) 7523-7556; (e) C. Wang, X. Huang, X. Liu, et al., Chin. Chem. Lett. 31 (2020) 677-680; (f) W. Li, L. Shu, Q. Wang, G. Li, Y. Shan, Chin. J. Org. Chem. 39 (2019) 1976-1982; (g) F. Wang, J. Wang, Y. Zhang, J. Yang, Chin. Chem. Lett. 31 (2020) 677-680; (h) C.X. He, Z.B. Jiang, H.Q. Cui, D.L. Yin, Chin. Chem. Lett. 27 (2016) 1036-1039; (i) X. Wu, J. Lei, Z.L. Song, Chin. Chem. Lett. 22 (2011) 306-309. |
| [2] |
(a) L. Chen, K. Chen, S. Zhu, Chem 4 (2018) 1208-1262; (b) A.V. Gulevich, A.S. Dudnik, N. Chernyak, V. Gevorgyan, Chem. Rev. 113 (2013) 3084-3213; (c) H. Wang, Y. Kuang, J. Wu, Asian J. Org. Chem. 1 (2012) 302-312; (d) A.L.S. Kumari, A.S. Reddy, K.C.K. Swamy, Org. Biomol. Chem. 14 (2016) 6651-6671; (e) L. Yang, L. Wei, J.P. Wan, Chem. Commun. 54 (2018) 7475-7478; (f) F. Peng, Q. Zhao, W. Huang, et al., Green Chem. 21 (2019) 6179-6186. |
| [3] |
(a) C.F. Zhu, S. Battah, X. Kong, et al., Bioorg. Med. Chem. Lett. 25 (2015) 558-561; (b) Q. Zhang, N.P. Bottingz, C. Kay, Chem. Commun. 47 (2011) 10596-10598; (c) M.A. Telpoukhovskaia, B.O. Patrick, C. Rodríguez-Rodríguez, C. Orvig, Bioorg. Med. Chem. Lett. 26 (2016) 1624-1628; (d) P.A. Wender, N. Buschmann, N.B. Cardin, et al., Nat. Chem. 3 (2011) 615-619. |
| [4] |
(a) K.E.O. Ylijoki, J.M. Stryker, Chem. Rev. 113 (2013) 2244-2266; (b) M.E. Garst, B.J. McBride, J.G. Douglass Ⅲ, Tetrahedron Lett. 24 (1983) 1675-1678. |
| [5] |
G. Mei, H. Yuan, Y. Gu, et al., Angew. Chem. Int. Ed. 53 (2014) 11051-11055. DOI:10.1002/anie.201406278 |
| [6] |
T. Cao, Y. Kong, K. Luo, L. Chen, S. Zhu, Angew. Chem. Int. Ed. 57 (2018) 8702-707. DOI:10.1002/anie.201801612 |
| [7] |
T. Cao, L. Chen, S. Zhu, Org. Lett. 21 (2019) 90-94. DOI:10.1021/acs.orglett.8b03519 |
| [8] |
(a) L. Zhang, T. Cao, H. Jiang, S. Zhu, Angew. Chem. Int. Ed. 59 (2020) 4670-4677; (b) Z. Guan, H. Zhu, Chin. J. Org. Chem. 40 (2020) 1398-1399. |
| [9] |
(a) N.T.T. Thuy, J.E. Lee, H.M. Yoo, N. Cho, J. Nat. Prod. 82 (2019) 3025-3032; (b) N. Al-Maharik, Nat. Prod. Rep. 36 (2019) 1156-1195; (c) A. Zafar, S. Singh, I. Naseem, Food Chem. Toxicol. 99 (2017) 149-161. |
 2020, Vol. 31
2020, Vol. 31 

