b Green Catalysis Center, College of Chemistry, Zhengzhou University, Zhengzhou 450001, China;
c State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China;
d Hunan Key Laboratory for the Design and Application of Actinide Complexes, University of South China, Hengyang 421001, China
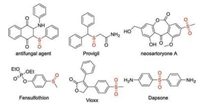
Sulfur-containing compounds are a class of important building blocks in organic synthesis and pharmaceuticals. For example, sulfoxides and sulfones are important moieties in the marketed drugs such as Provigil, Dapsone, Vioxx and Fensulfothion (Fig. 1). Selective oxidation of sulfides to the corresponding sulfoxides or sulfones is one of the most useful transformations for the construction of sulfoxides and sulfones. However, most of the previous reports rely on the strong oxidants (e.g., peroxides and inorganic salts) and excess amount of volatile solvents, which inextricably lead to large amount of waste. The past decades have witnessed the significant achievements of green chemistry. In this context, the exploration of highly controllable synthetic methods with sustainable reagents in green solvents under mild conditions is a longstanding goal for clean organic synthesis [1, 2].
|
Download:
|
| Fig. 1. Marketed drugs containing a sulfoxide or a sulfone. | |
The previous studies by our group [3, 4] and Jiang's group [5] have shown that the employment of appropriate solvents or catalytic conditions allows the precisely controlled selective oxidation of sulfides to the corresponding sulfoxides or sulfones. Despite these important achievements, however, volatile solvents (e.g., ethanol, acetonitrile) or external catalysts (e.g., UO2(OAc)2·2H2O, H3PO4) were still required to realize the switchable synthesis. Therefore, the development of catalyst-free oxidative procedure with green oxidant (ideally molecular oxygen) and sustainable solvents for the controllable oxidation of sulfides is highly desirable.
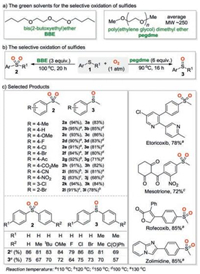
Considering that the reaction of polyether and molecular oxygen could generate the corresponding peroxide intermediates [6], it should be possible to apply an appropriate polyether as solvent and initiator to trigger the selective oxidation using molecular oxygen as oxidant. Very recently, the research group of Professor Wei-Min He [7] in Hunan University of Science and Engineering realized such a concept for the selective oxidation of sulfides to sulfoxides or sulfones in the presence of atmosphere pressure of molecular oxygen. In this work, a simple and catalyst-free solvent-controllable procedure was disclosed by using the inexpensive and easily available high-boiling-point ether as solvent. For example, when bis(2-butoxyethyl)ether (BBE) was applied as solvent, the oxidation of aromatic sulfides 1 under oxygen balloon at 100 ℃ for 20 h gave the corresponding sulfones 2 as products. On the other hand, when poly(ethylene glycol) dimethyl ether (PEGDME) (average molecular weight ~250) was employed as reaction media, the same reaction under 90 ℃ for 16 h provided the corresponding sulfoxides 3 as products in good yields (Schemes 1a and b). This solvent-controlled reaction showed broad substrate scope and excellent functional group compatibility. Overall, 38 examples of sulfones 2 and 35 examples of sulfoxides 3 were successfully synthesized. Notably, the sulfone- and sulfoxide-containing bioactive molecules including pesticides and pharma-ceuticals could be prepared through this selective oxidation procedure. For instance, the sulfone-containing artificial pharma-ceuticals (e.g., Etoricoxib, Mesotrione, Zolimidine and Rofecoxib) and sulfoxide-containing drug Sulindac were successfully prepared via the selective oxidation of precursors in good yields (Scheme 1c). Furthermore, the large-scale synthesis suggested that this eco-friendly protocol is potentially practical.
|
Download:
|
| Scheme 1. Controllable synthesis of sulfoxides or sulfones. | |
The investigation of reaction mechanism revealed that the singlet oxygen should be generated from the interaction of polyether and molecular oxygen. Moreover, the reaction could be inhibited by radical scavengers, suggesting the oxidation proceeds via a radical pathway. Both the singlet oxygen and the peroxyl radical were confirmed by the electron paramagnetic resonance (EPR) experiments. In addition, the 18O-labelled experiment demonstrated that the oxygen atoms of sulfoxides and sulfones are originally from the molecular oxygen. Based on those investigations, two possible reaction pathways were proposed for the oxidation. The singlet oxygen (1O2) or peroxide radical (polyether–OO·) were probably generated in the presence of polyether by heating. As shown in Scheme 2, the addition of the singlet oxygen to the sulfide 1 generates the intermediate A, which interacts with another 1 to form sulfoxide 3 (path a). Alternatively, the peroxide radical could oxidize the sulfide 1 via a single electron transfer to produce the radical cation intermediate B, which was converted into the persulfoxide radical C by reacting with oxygen. Then the intermediate C reacted with another 1 to afford sulfoxide 3 (path b). Furthermore, the sulfoxide 3 could be oxidized into the sulfone 2 through the similar process.

|
Download:
|
| Scheme 2. Proposed reaction pathway. | |
In conclusion, the He's group have developed a solvent-controlled selective oxidation of sulfides into sulfoxides or sulfones under molecular oxygen. Such findings provide a deeper insight into the polyether-initiated aerobic selective oxidation reaction. The application of the high-boiling-point polyether as an initiator and green media can eliminate the need of large quantities of additives and volatile solvents. These results will open new avenues in the field of green oxidative transformations due to the impressive simplicity and selectivity.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
| [1] |
K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30 (2019) 2304-2308. DOI:10.1016/j.cclet.2019.10.031 |
| [2] |
Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138. DOI:10.1016/j.cclet.2019.09.041 |
| [3] |
B. Yu, A.H. Liu, L.N. He, et al., Green Chem. 14 (2012) 957-962. DOI:10.1039/c2gc00027j |
| [4] |
B. Yu, Z.F. Diao, A.H. Liu, et al., Curr. Org. Synth. 11 (2014) 156-160. DOI:10.2174/1570179411999140304142430 |
| [5] |
Y. Li, S.A. Rizvi, D. Hu, et al., Angew. Chem. Int. Ed. 58 (2019) 13499-13506. DOI:10.1002/anie.201906080 |
| [6] |
J.Q. Wang, L.N. He, C.X. Miao, J. Gao, ChemSusChem 2 (2009) 755-760. DOI:10.1002/cssc.200900060 |
| [7] |
K.J. Liu, J.H. Deng, J. Yang, et al., Green Chem. 22 (2020) 433-438. DOI:10.1039/C9GC03713F |
 2020, Vol. 31
2020, Vol. 31 

