b Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China;
c School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
d School of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China
Molecular iodine is auseful reagent in modern organic synthesis, which could function as an iodinating reagent, a mild oxidant, or a mild Lewis acid. Compared with chlorine and bromine, iodine has the lowest homolytic dissociation energy (151 kJ/mol), which makes single-electron-transfer processes easy. Additionally, iodide anions are easily oxidized into molecular iodine by various oxidants. More importantly, iodine and its salts have a relatively low toxicity rendering them green reagents in the viewpoint of sustainable chemistry [1]. Due to the above-mentioned advantages, a plethora of iodine-catalyzed/mediated organic transformations have been developed in the past decades [2-5].
N-Heterocyclic1, 2, 3-benzotriazine is an important class of scaffold, which is extensively found in the fields of organic synthesis, pharmaceutical chemistry and material chemistry, due to its unique chemical activities and bioactivities [6]. Among them, 4-substituted-1, 2, 3-benzotriazines are the core structure of some significant molecules with anti-inflammatory and antitumor activities. Generally, the conventional strategies for the synthesis of 4-substituted-1, 2, 3-benzotriazines require the use of the highly active reactants such as diazonium salts and azides under harsh conditions. Consequently, the exploration of novel and efficient procedures for the construction of 4-substituted-1, 2, 3-benzotriazines from easily-handled-reagents under mild conditions is highly desirable and attractive.
Generally, rearrangement reaction could construct complicate molecules from easily available substrates. Therefore, rearrangement reaction could be an alternative strategy for the synthesis of 1, 2, 3-benzotriazines. Very recently, the research group of Professor Xiuling Cui and Professor Chao Pi from Zhengzhou University reported an elegant rearrangement approach for the synthesis of 1, 2, 3-benzotriazines through N-centered [1, 2]-rearrangement of 3-aminoindazoles with anilines without using diazonium salts or azides (Scheme 1) [7]. In this reaction, C=N and N—N bonds are simultaneously formed. Correspondingly, various N-aryl-1, 2, 3-benzotriazin-4(3H)-imines 3 were obtained in the presence of aryl amine 1 (0.12 mmol), 3-amino-1H-indazole 2 (0.1 mmol), I2 (10 mol%), tert-butyl hydroperoxide (TBHP, 2 equiv.) and tetra-butylammonium iodide (TBAI, 1.5 equiv.) in 1-propanol (2 mL) at 80 ℃ under air atmosphere for 12 h (Scheme 1a).
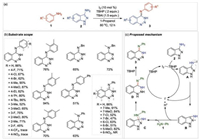
|
Download:
|
| Scheme 1. Iodine-catalyzed N-centered [1, 2]-rearrangement to synthesize 1, 2, 3-benzotriazines. | |
In the substrate scope, it was found that a large number of functional groups were compatible in this protocol, affording the corresponding1, 2, 3-benzotriazine products in moderate to excellent yields. Overall, 30 examples were successfully achieved. Moreover, the secondary amines including N-methyl-1-phenylmethanamine and N-methylaniline were well tolerated. Additionally, no steric effects were observed in this reaction. Similarly, the electronic effects were not obvious, while some substrates bearing strong electron-withdrawing groups (e.g., CF3, NO2) did not work in this procedure (Scheme 1b).
A plausible mechanism of the [1, 2]-rearrangement reaction was proposed as shown in Scheme 1c. First, in the present of TBHP and I2, the starting material 2a was iodinated to the intermediate A by releasing HI. Then the N-haloamine intermediate A was converted into the intermediate B via an intramolecular cyclization. Subsequently, the electron-rich nucleophile 1 attacked the intermediate B giving the intermediate C and a proton. Then in the ring expansion step, the intermediate C was transformed into D through a C—N bond cleavage. Finally, the desired product 3 was generated by oxidation of the intermediate D. Importantly, the catalyst molecular iodine should be regenerated from the oxidation of hydrogen iodide using TBHP as an oxidant.
In conclusion, the Cui's group has successfully developed a sustainable iodine-catalyzed N-centered [1, 2]-rearrangement reaction of 3-amino-1H-indazoles with aryl amines. A broad range of 1, 2, 3-benzotriazines were constructed in moderate to excellent yields with good functional group tolerance under the metal-free conditions. Significantly, the in situ formation of N-haloamine intermediate under the catalytic conditions is critical for this [1, 2]-rearrangement reaction. Such findings will open new routes for the application of molecular iodine in modern organic synthesis.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
| [1] |
P. Finkbeiner, B. Nachtsheim, Synthesis 45 (2013) 979-999. DOI:10.1055/s-0032-1318330 |
| [2] |
W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30 (2019) 2259-2262. DOI:10.1016/j.cclet.2019.06.052 |
| [3] |
Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361-1368. DOI:10.1016/j.cclet.2019.03.034 |
| [4] |
Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138. DOI:10.1016/j.cclet.2019.09.041 |
| [5] |
S. Du, C. Pi, T. Wan, Y. Wu, X. Cui, Adv. Synth. Catal. 361 (2019) 1766-1770. DOI:10.1002/adsc.201801433 |
| [6] |
G.M. Ziarani, M. Mostofi, N. Lashgari, Curr. Org. Chem. 22 (2018) 2717-2751. |
| [7] |
J. Ren, X. Yan, X. Cui, et al., Green Chem. 22 (2020) 265-269. DOI:10.1039/C9GC03567B |
 2020, Vol. 31
2020, Vol. 31 

