b Research Institute of Applied Chemistry, Shanxi University, Taiyuan 030006, China
Bio-thiols play important roles in living organisms which are often involved in physiological processes such as maintaining the homeostasis of redox, protein translation and folding, and signal transduction [1-4]. As a transit station for intracellular active sulfur species, cysteine (Cys) is particularly crucial. On the one hand, under aerobic conditions, Cys is decomposed into neurotransmitter taurine and gas signal molecule sulfur dioxide. On the other hand, in anaerobic conditions, Cys is catalyzed into gas signal molecule hydrogen sulfide [5-8]. These signal molecules also play important roles in human bodies, such as maintaining blood pressure and regulating vasodilation to regulate fibrinolysis and so on [9-11]. As the main task of Cys, regulating the intracellular redox balance depends on the concentration of Cys. So, the abnormality of Cys concentration is often related to diseases occurrences, such as cardiovascular and cerebrovascular diseases, Alzheimer's disease, depression, intestinal injury and even cancer [12-17]. And the normal intracellular Cys concentration should be 30–200 μmol/L [18, 19].
Correspondingly, as a cell state regulated by Cys, oxidative stress is one of the causes of many diseases, for instance, neurodegenerative diseases, cardiovascular diseases and diabetes [20-25]. Related papers pointed out that copper(II) can promote the production of reactive oxygen species (ROS) in cells through Fenton reaction [26, 27]. When a large amount of copper(II) is accumulated in cells, the level of ROS increase sharply and the redox balance in the cells is broken up, which results in a large amount of Cys will be consumed in order to maintain the redox homeostasis in the cells [28].
Because of unique advantages, fluorescent probes for all kinds of analytes were developed. In 2009, the first specifically fluorescent probe for detecting Cys has been developed by Peng's group, which promoted Cys fluorescent probe development [29-45]. However, small concentration changes of Cys during oxidative stress in cells raises a higher requirement for probes. Therefore, highly selective, sensitive and fast response fluorescent probes for Cys are still needed for practical application.
In this work, a fluorescent probe with a fast response was developed by linking coumarin derivatives containing α, β-unsaturated ketones to 7-nitro-1, 2, 3-benzoxadiazole (NBD) which has a large molar absorptivity and a high fluorescence quantum yield [46, 47]. The PETeffect made the system non-fluorescent. When the probe reacted with Cys, the bond between the coumarin derivative and the NBD was cut off, meanwhile a rapid rearrangement and reactive site passivation occurred. Then two fluorophores with the same emissionpeak are released, among them, strong fluorescence signal of NBD dominated. Thus, although the similar reaction occurred for Hcy, the rate of NBD derivative rearrangement was slow, in a short time, fluorescence signal was still weak. As for GSH, cleavage could occur, but no rearrange within the NBD molecule due to GSH with large volume. Because of strong fluorescent emission, this probe was successfully used in biological imaging about cell and zebrafish. More importantly, the probe was successfully used to evaluate the oxidative stress caused by copper(II) in living cells. At the same time, it was demonstrated that the addition of copper(II) can cause a decrease in Cys level.
The probe CN-1 was obtained and characterized through a series of experiments which can be found in Supporting information. The synthesis route of CN-1 was shown in Scheme 1.

|
Download:
|
| Scheme 1. The synthesis of the probe CN-1. | |
A series of spectral experiments were carried out. In PBS/DMSO (9/1, v/v, pH 7.4) containing 5 μmol/L of CN-1, Cys (0-100 μmol/L) was gradually added. As shown in Fig. 1a, with the addition of Cys, the fluorescence intensity at 555 nm was continuously enhanced. Under the same conditions, we performed kinetic experiments of CN-1 (5 μmol/L) with Cys (100 μmol/L), Hcy (100 μmol/L) and GSH (100 μmol/L). With the addition of Cys, the fluorescence intensity increased rapidly and basically reached a platform about 210 s as shown in Fig. 1b, but the time of Hcy and GSH was longer. The UV spectral experiment, selective test, detection limit and linear correlation were carried out as shown in Figs. S7–S10 (Supporting information). The above results indicated that the probe CN-1 can react with Cys in vitro and give a turn-on fluorescence signal.

|
Download:
|
| Fig. 1. (a) The fluorescence spectra of 100 μmol/L Cys added in PBS/DMSO (9/1, v/v) containing 5 μmol/L CN-1. (b) Time curve of CN-1 (5 μmol/L) with Cys (100 μmol/L), Hcy (100 μmol/L) and GSH (100 μmol/L) in PBS/DMSO (9/1, v/v, λex = 488 nm). | |
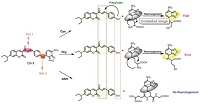
When the probe was designed, the strong nucleophilicity of -SH in Cys was supposed to be considered. It was speculated that the recognition mechanism of the probe is as follows (Scheme 2). Firstly, the -SH nucleophilic attacks on NBD successfully and replaces the coumarin moiety, which releases the coumarin fluorophore. In addition, the rearrangement occurred: The -SH attached to the NBD was replaced by the amino group on the Cys, also emitting strong yellow fluorescence. When the probe reacts with Cys, the bond between the coumarin derivative and the NBD was cut off and there is a rapid rearrangement occurred within the molecule of the NBD derivative. At this time, two fluorophores with the same emission peak are released. When reacts with Hcy, although the similar reaction occurs, the rate of NBD derivative rearrangement is slow. So, in a short time, there is only one fluorophore is allowed to be released. As for GSH, although the cut-off reaction can occur, since the GSH molecule is too large to rearrange within the NBD molecule, only one fluorophore is released. At the same time, due to the strong push electron effect of the -OH, the α, β-unsaturated double bond in the coumarin derivative is deactivated, and the thiol could not further react with it, so that the probe can keep a long emission wavelength.
|
Download:
|
| Scheme 2. The detection mechanism of CN-1. | |
In order to confirm the reaction mechanism, NMR and ESI-MS were employed. It could be seen from the NMR spectrum that there was no obvious peak disappearence or increase, but the position of the aromatic peak has changed and shifted to the high field (Fig. S4 in Supporting information). These changes are attributed to the interaction of Cys with NBD, which terminated the electron-withdrawing effect of NBD to coumarin. Next, we verified the result with high-resolution mass spectrometry. With the addition of Cys, it can be found that two peaks emerged at m/z 386.1368 and 283.0144 (Figs. S5 and S6 in Supporting information), which belong to the reaction products.
Prior to cell imaging, the toxicity of the probe to the cell was evaluated. As shown in Fig. S11 (Supporting information), the result showed that the probe CN-1 was low cytotoxic and could be used in cell experiments. Cell imaging was used to verify whether the probe CN-1 can detect Cys in Hela cells. As shown in Fig. S12 (Supporting information), first, the cells were incubated with the CN-1 (5 μmol/L) for 15 min, there was a weak yellow fluorescence. Then, NEM (2 mmol/L) was used to incubate another batch of cells for 20 min, then were treated with CN-1 (5 μmol/L), the yellow channel was still no fluorescent emission. After incubated with CN-1, the cells incubated with Cys (100 μmol/L), Hcy (100 μmol/L), and GSH (100 μmol/L) respectively (15 min), only incubated with Cys showed a significantly strong yellow fluorescence (Fig. S13 in Supporting information). Therefore, CN-1 can specifically image exogenous/endogenous Cys in living cells.
According to the relevant report, Cys concentration changes are often associated with diseases. In order to assess whether the probe can detect changes in cysteine concentration, different concentrations of Cys were labeled in Hela cells. As shown in Fig. S14 (Supporting information), as the concentration of exogenous Cys increases, the fluorescent intensity in the yellow channel increased. Those results showed that probe CN-1 can monitor the changes in Cys concentration in the cells.
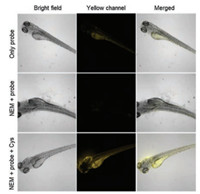
Furthermore, we applied probe imaging in zebrafish. In Fig. 2, CN-1 (10 μmol/L, 15 min) was directly incubated with untreated zebrafish, a strong fluorescent signal was obtained in the yellow channel. However, when the probe was incubated in NEM-pretreated zebrafish, the fluorescence signal intensity was significantly reduced. After Cys reperfusion of NEM and probe pretreated zebrafish, the fluorescence intensity was enhanced again in the yellow channel. It was revealed that CN-1 can specifically image endogenous/exogenous Cys in zebrafish.
|
Download:
|
| Fig. 2. Bioimaging of CN-1 detecting endogenous/exogenous Cys in a 5 days old zebrafish: Zebrafish with CN-1 (10 μmol/L) (top); NEM (2 mmol/L) pre-treating zebrafish with CN-1 (10 μmol/L) (middle); NEM (2 mmol/L) pre-treating zebrafish with CN-1 (10 μmol/L) and Cys(200 μmol/L) (bottom). Yellowchannel, λex = 488 nm, λex= 520-580 nm. | |
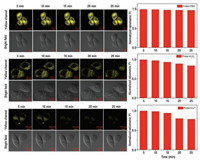
H2O2, a representative ROS, is the preferred candidate for evaluating the performance of CN-1 for imaging the redox dynamic in Hela cells. As shown in Fig. 3, the fluorescent emission of CN-1 (10 μmol/L, 15 min) in Hela cells in the yellow channel within 25 min almost no changed. The Hela cells with CN-1 (10 μmol/L, 15 min) which were incubated H2O2 (2 mmol/L) showed a gradual decrease in fluorescence in the following 25 min. The phenomenon indicated that CN-1 is a good candidate to image the redox dynamic in Hela cells. CuSO4 was used as a copper source, with the time increasing, the fluorescence intensity in the cells decreased significantly. All of the results might be that copper(II) stimulated the cells to produce ROS, and a large amount of ROS undergo redox reactions with Cys, resulting in a decrease in the amount of Cys in the cells. So, the fluorescence in the yellow channel cells is gradually reduced.
|
Download:
|
| Fig. 3. Time-dependent cells-imaging with CN-1 (10 μmol/L) and PBS (10 mmol/L)/ H2O2 (2 mmol/L)/ Cu2+ (2 mmol/L). Yellow channel, λex = 488 nm, λex = 520–580 nm. Scale bars: 10 μm. | |
In conclusion, a selective and sensitive probe CN-1 for Cys was designed and synthesized and applied to the detection of Cys in vivo and image the oxidative stress induced by copper(II) ions in living cells. During the recognition process, the CN-1 showed a "turn-on" fluorescence response to Cys in the PBS-DMSO (9/1, v/v, pH 7.4). The detection limit of CN-1 for detecting Cys was calculated to 0.44 μmol/L. At the same time, CN-1 can detect endogenous/exogenous Cys in vivo. The experiments in cells indicated that CN-1 can be used to image and evaluate the redox stress induced by copper(II) ions in Hela cells. In summary, due to its excellent properties, the probe has great potential for monitoring the redox stress induced by heavy-metal ions to diagnose related diseases.
Declaration of competing interestWe have no any interest conflict.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21775096, 21907062), One Hundred People Plan of Shanxi Province, Shanxi Province "1331 Project" Key Innovation Team Construction Plan Cultivation Team (No. 2018-CT-1), 2018 Xiangyuan County Solid Waste Comprehensive Utilization Science and Technology Project (No. 2018XYSDJS-05), the Shanxi Province Foundation for Selected (2019), Innovative Talents of Higher Education Institutions of Shanxi (2019), Scientific and Technologi-cal Innovation Programs of Higher Education Institutions in Shanxi (No. 2019L0031), Key R & D Program of Shanxi Province (No. 201903D421069), Natural Science Foundation of Shanxi Province of China (No. 201901D111015), Shanxi Province Foundation for Returness (No. 2017-026), Program for the Innovative Talents of Higher Education Institutions of Shanxi (2019), Shanxi Collabora-tive Innovation Center of High Value-added Utilization of Coal-related Wastes (No. 2015-10-B3) and Scientific Instrument Center of Shanxi University (No. 201512).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.001.
| [1] |
S. Shahrokhian, Anal. Chem. 73 (2001) 5972-5978. DOI:10.1021/ac010541m |
| [2] |
S.Y. Zhang, C.N. Ong, H.M. Shen, Cancer Lett. 208 (2004) 143-153. DOI:10.1016/j.canlet.2003.11.028 |
| [3] |
T.P. Dalton, H.G. Shertzer, A. Puga, Annu. Rev. Pharmacol. Toxicol. 39 (1999) 67-101. DOI:10.1146/annurev.pharmtox.39.1.67 |
| [4] |
X.M. Shao, R.X. Kang, Y.L. Zhang, et al., Anal. Chem. 87 (2015) 399-405. DOI:10.1021/ac5028947 |
| [5] |
D.J. Lefer, Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 17907-17908. DOI:10.1073/pnas.0709010104 |
| [6] |
N. Shibuya, S. Koike, M. Tanaka, et al., Nat. Commun. 4 (2013) 1366-1373. DOI:10.1038/ncomms2371 |
| [7] |
C. Gordon, H. Bradley, Lancet 339 (1992) 25-26. DOI:10.1016/0140-6736(92)90144-R |
| [8] |
M.H. Stipanuk, P.W. Beck, Biochem. J. 206 (1982) 267-277. DOI:10.1042/bj2060267 |
| [9] |
H. Kimura, N. Shibuya, Y. Kimura, Antioxid. Redox Signal. 17 (2012) 45-57. DOI:10.1089/ars.2011.4345 |
| [10] |
Jackson-Weaver O., J.M. Osmond, M. Riddle, et al., Am. J. Physiol. Heart Circ. Physiol. 304 (2013) 1446-1454. DOI:10.1152/ajpheart.00506.2012 |
| [11] |
P. Ariyaratnam, M. Loubani, A.H. Morice, Microvasc. Res. 90 (2013) 135-137. DOI:10.1016/j.mvr.2013.09.002 |
| [12] |
Y.M. Go, D.P. Jones, Free Radic. Biol. Med. 50 (2011) 495-509. DOI:10.1016/j.freeradbiomed.2010.11.029 |
| [13] |
El-Khairy L., P.M. Ueland, H. Refsum, I.M. Graham, S.E. Vollset, Circulation 103 (2001) 2544-2549. DOI:10.1161/01.CIR.103.21.2544 |
| [14] |
A.C. McQuilken, Y. Ha, K.D. Sutherlin, et al., J. Am. Chem. Soc. 135 (2013) 14024-14027. DOI:10.1021/ja4064487 |
| [15] |
J.M. Zhuo, H. Wang, D. Pratico, Trends Pharmacol. Sci. 32 (2011) 562-571. DOI:10.1016/j.tips.2011.05.003 |
| [16] |
Bauchart-Thevret C., B. Stoll, S. Chacko, D.G. Burrin, Am. J. Physiol. Endocrinol. Metab. 296 (2009) 1239-1250. DOI:10.1152/ajpendo.91021.2008 |
| [17] |
M.H. Stipanuk, J.E. Dominy, J.I. Lee, R.M. Coloso, J. Nutr. 136 (2006) 1652-1659. DOI:10.1093/jn/136.6.1652S |
| [18] |
J.C. Lim, L. Lam, B. Li, Exp. Eye Res. 116 (2013) 219-226. DOI:10.1016/j.exer.2013.09.002 |
| [19] |
E.C. Lien, C.A. Lyssiotis, A. Juvekar, et al., Nat. Cell Biol. 18 (2016) 572-578. DOI:10.1038/ncb3341 |
| [20] |
B.N. Tripathi, S.K. Mehta, A. Amar, J.P. Gaur, Chemosphere 62 (2006) 538-544. DOI:10.1016/j.chemosphere.2005.06.031 |
| [21] |
G.N. Kim, Y.I. Kwon, H.D. Jang, Toxicol. In Vitro 25 (2011) 138-144. DOI:10.1016/j.tiv.2010.10.005 |
| [22] |
X.K. Tian, Y. Zhao, Y. Li, C. Yang, Z.X. Zhou, Sens. Actuators B:Chem. 247 (2017) 139-145. DOI:10.1016/j.snb.2017.03.011 |
| [23] |
Rodri'guez-Rodri'guez C., M. Telpoukhovskaia, C. Orvig, Coord. Chem. Rev. 256 (2012) 2308-2332. DOI:10.1016/j.ccr.2012.03.008 |
| [24] |
F.M. Zhou, G.L. Millhauser, Coord. Chem. Rev. 256 (2012) 2285-2296. DOI:10.1016/j.ccr.2012.04.035 |
| [25] |
J. Li, Y.K. Yue, F.J. Huo, C.X. Yin, Dyes Pigm. 164 (2019) 335-340. DOI:10.1016/j.dyepig.2019.01.045 |
| [26] |
G.T. Sfrazzetto, C. Satriano, G.A. Tomaselli, E. Rizzarelli, Coord. Chem. Rev. 311 (2016) 125-167. DOI:10.1016/j.ccr.2015.11.012 |
| [27] |
A.N. Pham, G.W. Xing, C.J. Miller, T.D. Waite, J. Catal. 301 (2013) 54-64. DOI:10.1016/j.jcat.2013.01.025 |
| [28] |
M. Hepel, S. Andreescu, ACS Symposium Series, ACS, Washington, DC (2015). |
| [29] |
H.L. Li, J.L. Fan, J.Y. Wang, et al., Chem. Commun. 39 (2009) 5904-5906. |
| [30] |
Y. Wen, F.J. Huo, C.X. Yin, Chin. Chem. Lett. 30 (2019) 1834-1842. DOI:10.1016/j.cclet.2019.07.006 |
| [31] |
L. Song, L.M. Ma, Q. Sun, et al., Chin. Chem. Lett. 27 (2016) 330-334. DOI:10.1016/j.cclet.2015.12.012 |
| [32] |
Z.H. Fu, X. Han, Y.L. Shao, et al., Anal. Chem. 89 (2017) 1937-1944. DOI:10.1021/acs.analchem.6b04431 |
| [33] |
M.Y. Li, P.C. Cui, K. Li, et al., Chin. Chem. Lett. 29 (2018) 992-994. DOI:10.1016/j.cclet.2017.11.011 |
| [34] |
X.X. Xie, C.X. Yin, Y.K. Yue, F.J. Huo, Sens. Actuators B:Chem. 267 (2018) 76-82. DOI:10.1016/j.snb.2018.04.030 |
| [35] |
H.H. Song, J.L. Zhang, X. Wang, et al., Sens. Actuators B:Chem. 259 (2018) 233-240. DOI:10.1016/j.snb.2017.12.010 |
| [36] |
L. Yang, H.Q. Xiong, Y.N. Su, et al., Chin. Chem. Lett. 30 (2019) 563-565. DOI:10.1016/j.cclet.2018.12.017 |
| [37] |
K.Y. Liu, H.M. Shang, X.Q. Kong, W.Y. Lin, J. Mater. Chem. B 5 (2017) 3836-3841. DOI:10.1039/C7TB00187H |
| [38] |
Y.F. Kang, L.Y. Niu, Q.Z. Yang, Chin. Chem. Lett. 30 (2019) 1791-1798. DOI:10.1016/j.cclet.2019.08.013 |
| [39] |
B.X. Zhang, H.J. Zhang, M. Zhong, et al., Chin. Chem. Lett. 31 (2020) 133-135. DOI:10.1016/j.cclet.2019.05.061 |
| [40] |
L.J. Tang, D. Xu, M.Y. Tian, J. Lumin. 208 (2019) 502-508. DOI:10.1016/j.jlumin.2019.01.022 |
| [41] |
Q. Hu, C.Q. Qin, L. Huang, et al., Dyes Pigment. 149 (2018) 253-260. DOI:10.1016/j.dyepig.2017.10.002 |
| [42] |
L.M. Zhu, J.C. Xu, Z. Sun, et al., Chem. Commun. 51 (2015) 1154-1156. DOI:10.1039/C4CC08258C |
| [43] |
J.C. Xu, H.Q. Yuan, L.T. Zeng, G.M. Bao, Chin. Chem. Lett. 29 (2018) 1456-1464. DOI:10.1016/j.cclet.2018.08.012 |
| [44] |
X.J. Liu, W.Y. Zhang, C.X. Li, et al., RSC Adv. 5 (2015) 4941-4946. DOI:10.1039/C4RA13262A |
| [45] |
X. Zhou, X. Jin, G. Sun, D. Li, X. Wu, Chem. Commun. 48 (2012) 8793-8795. DOI:10.1039/c2cc33971d |
| [46] |
T. Toyooka, T. Suzuki, Y. Saito, Analyst 10 (1989) 1233-1240. |
| [47] |
S. Uchiyama, T. Santa, T. Fukushima, J. Chem. Soc. Perkin Trans. 10 (1998) 2165-2174. |
 2020, Vol. 31
2020, Vol. 31 

