Room temperature phosphorescence (RTP) of pure organic molecules has been rapidly developed in recent years due to their long-lifetime emission [1-4] and potential applications in organic light-emitting diodes [5-7], molecular sensing [8, 9], time-resolved bioimaging [10-13] and security technology [14-17]. In order to activate the phosphorescence emission of RTP compounds, two vital factors should be considered: (i) promoting singlet-triplet intersystem crossing (ISC); (ii) suppressing the non-radiative relaxation pathways [18-21]. In general, most of the design strategies for achieving RTP emission are introducing heavy halogen atoms or aromatic carbonyl groups to organic compounds to facilitate ISC process and confining the RTP compounds into a rigid environment to minimize the intermolecular and intramolecular motions, including polymer matrix [22-25], host-guest interaction [26-28], crystal formation [29-34] and doped thin films [35, 36]. However, some of the stringent molecular states, such as crystal structures, on the one hand, have effectively promoted their RTP performance, but to a certain extent also limit their application for the strict growth conditions and limited repeatability, such as light-emitting devices and biocompatibility. As to thin films, some are made of host and guest. These two parts should match with each other in energy levels, thus causing limitations in the design of molecular structures. Besides, host matrix may change the photophysical properties of the guest RTP emitter. Compared with them, non-crystalline organic small molecules are rarely plagued by these problems. Thus, it is important to develop non-crystalline organic small molecules with RTP emission.
The luminescence of proteins has attracted great interest due to the importance of its basic theoretical research and its broad application prospects [37, 38]. The traditional view is that the luminescence of proteins, especially RTP, is mainly derived from three aromatic amino acids: tryptophan, tyrosine and phenylalanine [39]. Recently, our group has reported a series of noncrystalline organic small molecular compounds with efficient RTP emission properties through conveniently grafting phosphor moieties to β-cyclodextrin, attributed to the strong intermolecular hydrogen bonding between cyclodextrins [40, 41]. Thus, there are enough reasons for us to presume that there is a rigid environment which can enhance RTP emission due to the formation of hydrogen bond of amino acids in aggregation state.
Herein, six non-crystalline organic small molecules with RTP emission are reported, by modifying different phosphors, with heavy bromine atoms to facilitate the intersystem crossing process from excited singlet to triplet, onto diphenylalanine and phenylalanine derivatives. It is a novel strategy for the realization of noncrystalline organic small molecules with RTP emission. Benefiting from the skeletal structure of the amino acid derivatives, there are intermolecular hydrogen bond formation. As is known to all, abundant hydrogen-bonding network could not only limit the intermolecular motions efficiently due to its rigidification effect, but shield from quenchers as well, thus enhancing their RTP performances.
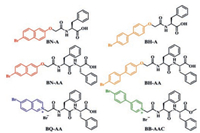
For proving the feasibility of the aromatic amino acid system which can emit RTP, we synthesized six different amino acid derivatives, namely, bromonaphthol-phenylalanine (BN-A), bromohydroxybiphenyl-phenylalanine (BH-A), bromonaphthol-diphenylalanine (BN-AA), bromohydroxybiphenyl-diphenylalanine (BH-AA), bromoisoquinoline-diphenylalanine (BQ-AA) and bromophenylpyridine-diphenylalaninate (BB-AAC) (Scheme 1). The synthetic routes were shown in Schemes S1-S6 (Supporting information). The intermediates and final products of right structures with high purity are characterized via 1H NMR, 13C NMR and high-resolution mass spectra (Figs. S1-S33 in Supporting information). Through incorporation of phosphorescentfunctional groups onto amino acid skeletal structures, six organic small molecules with RTP emission property were prepared successfully. First, the heavy halogen atom bromine in the phosphor unit could promote the process of intersystem crossing to some extent. In addition, the RTP emission can be enhanced by intermolecular hydrogen bond formation with rigidification effect.
|
Download:
|
| Scheme 1. Structures of six amino acid derivatives, BN-A, BH-A, BN-AA, BH-AA, BQ-AA and BB-AAC. The configurations of six amino acid derivatives are all L-enantiomers. | |
The six amino acid derivatives were found to have no obvious fluorescence emission in solid state except BH-AA, for the heavy atom quenching effect of bromine (Fig. S35 in Supporting information), while obvious RTP emission could be observed from their solid powder as anticipated. As shown in Fig. 1, the RTP emission spectrum revealed that BH-A powder has a clear wide peak at about 500 nm, and the lifetime is 370 μs (Fig. S36b in Supporting information), which can be proved as phosphorescence emission. The phosphorescent quantum yield of BH-A is 1.7%. This is most likely attributed to the quantity of the amino and carboxyl groups in amino acid, which can form hydrogen-bonding interaction among adjacent molecules, is not as much as the quantity of hydroxyl groups in cyclodextrin which was often used in our previous work [40, 41]. Therefore, the rigid network for suppressing non-radiative process is not so powerful and it could only shield from a part of oxygen in surrounding microenvironment and limit the motion of molecules to some extent. Under 365 nm UV light excitation, light could be seen clearly by naked eyes (Fig. S37b in Supporting information). The other five compounds demonstrated similar properties with BH-A and their photophysical data are collected in Table 1. Their solid absorption curves are also similar (Fig. S34 in Supporting information), with maximum absorption distributing in the range of 250–400 nm. As shown in Fig. 1b, the maximum excitation of BH-A is about 300 nm, thus the Stokes shift of RTP emission reached about 200 nm by comparing with its emission spectrum. The Stokes shifts of the other five compounds are 200 nm for BN-A, 215 nm for BN-AA, 200 nm for BH-AA, 267 nm for BQ-AA and 185 nm for BB-AAC, respectively (Table 1), which further testified their RTP emission characteristics. The lifetimes of the emission peaks were also detected to confirm the RTP emission (Fig. S36 in Supporting information). Thus, six organic small molecules with RTP emission by using facile synthetic methods were obtained.

|
Download:
|
| Fig. 1. RTP excitation (black line) and emission (red line) spectra of (a) BN-A, (b) BH-A, (c) BN-AA, (d) BH-AA, (e) BQ-AA and (f) BB-AAC. Phosphorescence mode: BN-AA tested with excitation slim=20 nm; emission slim=20 nm. BN-A, BH-A, BH-AA, BQ-AA and BB-AAC tested with excitation slim=10 nm; emission slim=10 nm. | |
|
|
Table 1 Photophysical data of six amino acid derivatives in solid state. |
To investigate the microstructures of the six compounds, X-ray powder diffraction (XRD) was also carried out. As shown in Fig. 2, there is a wide peak from BQ-AA and the other five compounds show no obvious crystal diffraction peak from the results, representing the basically non-crystalline state of these solid powder. Therefore, it is so interesting to observe RTP emission in non-crystalline state, for it is infrequent in small molecular systems, which confirms the feasibility of this strategy greatly. It is hypothesized that the skeletal structures of diphenylalanine and phenylalanine play a key role in determining the molecular state, thus making these solid powder exhibit non-crystalline state.

|
Download:
|
| Fig. 2. XRD patterns of BN-A, BH-A, BN-AA, BH-AA, BQ-AA and BB-AAC solid powder. | |
To develop non-crystalline organic small molecules with RTP emission property remains a formidable challenge, since the energy of the excited triplet states of molecules could be easily lost via thermally vibrational and collisional process or be quenched by oxygen in surrounding microenvironment, especially in non-crystalline state. It has been reported that the hydrogen-bonding interaction dominates the enhancement of RTP in some kinds of RTP emission systems [40-45]. Thus, we hypothesized that there is a rigid environment composed of hydrogen-bonding interaction among the molecules, which restrain the vibration and rotation of the phosphors, making these six molecules emit RTP emission in non-crystalline state. In this work, it was reasonable to assume that the amino acid moiety only played a role in providing a rigid hydrogen-bonding network with rigidification effect for RTP emission, rather than promoting the process of ISC. To confirm the hypothesis of the effect of amino acid unit on RTP emission in this system, b3lyp/6-311G* calculations were employed to study the frontier molecular orbits of the amino acid derivatives and the phosphors. As shown in Fig. 3, the HOMO or LUMO orbits of the amino acid derivatives were almost the same as the ones of their corresponding phosphors, demonstrating that there was no electron transfer process between the amino acid moiety and phosphors. That is to say, the amino acid moiety did not have an impact on the ISC process of the phosphors except forming a rigid hydrogen-bonding network. In addition, the heavy halogen atom bromine in the phosphor unit could facilitate the process of intersystem crossing by promoting spin-orbit coupling. As shown in Table 1, the quantum yields of phenylalanine derivatives are higher than diphenylalanine derivatives. Compared with diphenylalanine, the skeleton of phenylalanine is somewhat less flexible, with which the corresponding compounds are more rigid, making the RTP emission intensity much stronger.

|
Download:
|
| Fig. 3. The comparison diagram between calculated HOMO and LUMO for optimized S0 states of amino acid derivatives and their corresponding phosphors. | |
In conclusion, six non-crystalline organic small molecules, BN-A, BH-A, BN-AA, BH-AA, BQ-AA and BB-AAC with RTP emission were designed and synthesized facilely. The skeletal structure of the amino acid derivatives and the rigid environment composed of hydrogen-bonding interaction with rigidification effect among molecules restrain the vibration and rotation of the phosphors, giving these small molecules the ability to emit RTP emission in non-crystalline state. This study supplies a new and efficient strategy to facilely construct RTP from non-crystalline organic small molecules, especially the aromatic amino acid derivatives, and helps to enrich the organic RTP material families as well.
Declaration of competing interestThe authors declare no competing financial interest.
AcknowledgmentsWe gratefully acknowledge the financial support from the National Natural Science Foundation of China (NSFC, Nos. 21788102, 21722603 and 21871083), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX03), 'Shu Guang' Project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No. 19SG26), the Innovation Program of Shanghai Municipal Education Commission (No. 2017-01-07-00-02-E00010) and Fundamental Research Funds for the Central Universities.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.05.015.
| [1] |
B. Lu, S.Y. Liu, D.P. Yan, Chin. Chem. Lett. 30 (2019) 1908-1922. DOI:10.1016/j.cclet.2019.09.012 |
| [2] |
D.P. Yan, D.G. Evans, Mater. Horiz. 1 (2014) 46-57. DOI:10.1039/C3MH00023K |
| [3] |
B. Zhou, D.P. Yan, Angew. Chem. Int. Ed. 58 (2019) 15128-15135. DOI:10.1002/anie.201909760 |
| [4] |
B. Zhou, D.P. Yan, Adv. Funct. Mater. 29 (2019) 1807599. DOI:10.1002/adfm.201807599 |
| [5] |
H. Wang, X. Lv, P. Wang, et al., Chin. Chem. Lett. 29 (2018) 471-474. DOI:10.1016/j.cclet.2017.07.025 |
| [6] |
X.Z. Song, D.D. Zhang, T.Y. Huang, et al., Sci. China Chem. 61 (2018) 836-843. DOI:10.1007/s11426-018-9242-1 |
| [7] |
B.W. Li, X.A. Song, X. Jiang, et al., Chin. Chem. Lett. 31 (2020) 1188-1192. DOI:10.1016/j.cclet.2019.06.033 |
| [8] |
Y. Liu, G. Zhan, Z.W. Liu, et al., Chin. Chem. Lett. 27 (2016) 1231-1240. DOI:10.1016/j.cclet.2016.06.029 |
| [9] |
B.I. Ipe, K. Yoosaf, K.G. Thomas, J. Am. Chem. Soc. 128 (2006) 1907-1913. DOI:10.1021/ja054347j |
| [10] |
H.F. Shi, L. Zou, K.W. Huang, et al., ACS Appl. Mater. Interfaces 11 (2019) 18103-18110. DOI:10.1021/acsami.9b01615 |
| [11] |
J. Yang, X. Zhen, B. Wang, et al., Nat. Commun. 9 (2018) 840. DOI:10.1038/s41467-018-03236-6 |
| [12] |
R. Gao, X. Mei, D. Yan, et al., Nat. Commun. 9 (2018) 2798. DOI:10.1038/s41467-018-05223-3 |
| [13] |
N. Gan, H. Shi, Z. An, et al., Adv. Funct. Mater. 28 (2018) 1802657. DOI:10.1002/adfm.201802657 |
| [14] |
Q.X. Xiong, C. Xu, X. Ma, et al., Chin. Chem. Lett. 30 (2019) 1387-1389. DOI:10.1016/j.cclet.2019.04.010 |
| [15] |
T. Zhang, H. Chen, X. Ma, et al., Ind. Eng. Chem. Res. 56 (2017) 3123-3128. DOI:10.1021/acs.iecr.7b00149 |
| [16] |
Y.N. Zhang, J.F. Zhao, C.X. Zhu, et al., Chin. Chem. Lett. 30 (2019) 1974-1978. DOI:10.1016/j.cclet.2019.09.005 |
| [17] |
J.X. Wang, Y.G. Fang, C.X. Li, et al., Angew. Chem. Int. Ed. 59 (2020) 10032-10036. DOI:10.1002/anie.202001141 |
| [18] |
Z.H. He, W.B. Li, G. Chen, et al., Chin. Chem. Lett. 30 (2019) 933-936. DOI:10.1016/j.cclet.2019.03.015 |
| [19] |
X.Y. Fang, D.P. Yan, Sci. China Chem. 61 (2018) 397-401. DOI:10.1007/s11426-017-9183-9 |
| [20] |
Y.Y. Gong, G. Chen, Q. Peng, et al., Adv. Mater. 27 (2015) 6195-6201. DOI:10.1002/adma.201502442 |
| [21] |
M.S. Kwon, D. Lee, S. Seo, et al., Angew. Chem. Int. Ed. 53 (2014) 11177-11181. DOI:10.1002/anie.201404490 |
| [22] |
B. Zhou, D.P. Yan, Sci. China Chem. 62 (2019) 291-292. DOI:10.1007/s11426-018-9389-7 |
| [23] |
X. Wang, Y. Xu, X. Ma, et al., Ind. Eng. Chem. Res. 57 (2018) 2866-2872. DOI:10.1021/acs.iecr.7b04759 |
| [24] |
X. Ma, C. Xu, J. Wang, et al., Angew. Chem. Int. Ed. 57 (2018) 10854-10858. DOI:10.1002/anie.201803947 |
| [25] |
H. Chen, X.Y. Yao, X. Ma, et al., Adv. Opt. Mater. 4 (2016) 1397-1401. DOI:10.1002/adom.201600427 |
| [26] |
G.J. Qu, Y.P. Zhang, X. Ma, Chin. Chem. Lett. 30 (2019) 1809-1814. DOI:10.1016/j.cclet.2019.07.042 |
| [27] |
X. Ma, J. Wang, H. Tian, Acc. Chem. Res. 52 (2019) 738-748. DOI:10.1021/acs.accounts.8b00620 |
| [28] |
Y.F. Gong, H. Chen, X. Ma, et al., ChemPhysChem 17 (2016) 1934-1938. DOI:10.1002/cphc.201500901 |
| [29] |
X.F. Wang, W.J. Guo, H.Y. Xiao, et al., Adv. Funct. Mater. 30 (2020) 1907282. DOI:10.1002/adfm.201907282 |
| [30] |
W.J. Luo, Y.R. Zhang, Y.Y. Gong, et al., Chin. Chem. Lett. 29 (2018) 1533-1536. DOI:10.1016/j.cclet.2018.08.001 |
| [31] |
J.A. Li, J.H. Zhou, Z. Mao, et al., Angew. Chem. Int. Ed. 57 (2018) 6449-6453. DOI:10.1002/anie.201800762 |
| [32] |
X.H. Chen, W.J. Luo, H.L. Ma, et al., Sci. China Chem. 61 (2018) 351-359. DOI:10.1007/s11426-017-9114-4 |
| [33] |
Z.H. He, W.B. Li, G. Chen, et al., Chin. Chem. Lett. 30 (2019) 933-936. DOI:10.1016/j.cclet.2019.03.015 |
| [34] |
X. Wang, N. Gan, M.X. Gu, et al., Chin. Chem. Lett. 30 (2019) 1935-1938. DOI:10.1016/j.cclet.2018.12.023 |
| [35] |
N.A. Kukhta, R. Huang, A.S. Batsanov, et al., J. Phys. Chem. C 123 (2019) 26536-26546. DOI:10.1021/acs.jpcc.9b08238 |
| [36] |
R.J. Huang, J.S. Ward, N.A. Kukhta, et al., J. Mater. Chem. C 6 (2018) 9238-9247. DOI:10.1039/C8TC02987C |
| [37] |
Q. Zhou, B.Y. Cao, C.X. Zhu, et al., Small 12 (2016) 6586-6592. DOI:10.1002/smll.201601545 |
| [38] |
Y.Y. Gong, Y.Q. Tan, J. Mei, et al., Sci. China Chem. 56 (2013) 1178-1182. DOI:10.1007/s11426-013-4923-8 |
| [39] |
A.H. Maki, J.A. Zuclich, Top. Curr. Chem. 54 (1975) 115-163. |
| [40] |
C.X. Zhao, Y.H. Jin, X. Ma, et al., Chem. Commun. 55 (2019) 5355-5358. DOI:10.1039/C9CC01594A |
| [41] |
D.F. Li, F.F. Lu, X. Ma, et al., J. Am. Chem. Soc. 140 (2018) 1916-1923. DOI:10.1021/jacs.7b12800 |
| [42] |
Y. Su, S.Z.F. Phua, Y.B. Li, et al., Sci. Adv. 4 (2018) eaas9732. DOI:10.1126/sciadv.aas9732 |
| [43] |
Q. Wang, M. Cheng, J.L. Jiang, et al., Chin. Chem. Lett. 28 (2017) 793-797. DOI:10.1016/j.cclet.2017.02.008 |
| [44] |
T. Zhang, C.Y. Wang, X. Ma, Ind. Eng. Chem. Res. 58 (2019) 7778-7785. DOI:10.1021/acs.iecr.9b00910 |
| [45] |
D.S. Wang, X. Wang, X. Ma, et al., Sci. China Chem. 62 (2019) 430-433. DOI:10.1007/s11426-018-9383-2 |
 2020, Vol. 31
2020, Vol. 31 


