b School of Automation, Xi'an University of Posts and Telecommunications, Xi'an 710121, China;
c Department of Chemistry, University of Bath, Bath, BA2 7AY, United Kingdom
Biological hypochlorous acid (HOCl) is an interesting type of reactive oxygen species (ROS) that plays crucial roles in beneficial immunoregulatory response and pathogenic oxidative stress injuries [1-3]. At the cellular level, HOCl is endogenously generated by a myeloperoxidase (MPO)-catalyzed oxidation reaction between chloride ions (Cl-) and hydrogen peroxide (H2O2) [3, 4]. Although HOCl with antimicrobial activity is valuable for host defense during microbial invasion [5], the generation of excessive HOCl leads to many diseases related to oxidative stress damage including aging [6], cancer [7], inflammation [8] and neuro-degenerative disorders [9]. Therefore, highly selective and sensitive tools for the detection of HOCl levels in complex biosystems are of particular interest for accurate clinical diagnosis. For this purpose, methods, including chromatographic, electrochemical, colorimetric and fluorescence, have been developed, among which, nondestructive fluorescence methods based on small-molecule probes are the most desirable for real-time and in situ detection [10].
In recent years, a number of small-molecule fluorescent probes have been developed for the detection of HOCl in vivo [5, 10, 6-23]. Some of them have been designed based on the C=N unit [20, 6-23]. Generally, these types of probes contain acyclic C=N bonds and are non-fluorescent due toisomerization quenching.WhenHOCl reacts with the C=N, the isomerization is removed, thus leading to a turnon fluorescence response. According to the reaction mechanism reported in the literature [21], HOCl is able to react with the C=N bond to form formyl or carboxyl compounds via hydrolysis and oxidization. Based on this oxidation-leaving group approach, many HOCl probes have been developed [20, 21], but significant background interference and lack of selectivity have limited their application in complex biosystems.
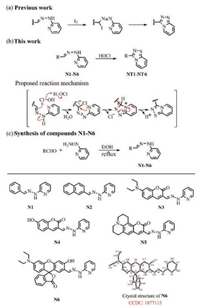
The aforementioned concerns encouraged us to develop a powerful new strategy in order to improve the performance of this type of probe. According to a previous report [24], I+ from the decomposition of I2 can attack the C=N bond of 2-pyridylhydrazone thereby inducing 2-pyridylhydrazone to form 1, 2, 4-triazolo[4, 3-a]pyridine through a cyclization reaction (Scheme 1a). We speculated that HOCl containing a positive monovalent chlorine should also be able to convert 2-pyridylhydrazone into 1, 2, 4-triazolo[4, 3-a]pyridine. To prove our hypothesis, we synthesized compounds N1 and N2 by the reaction of 2-hydrazinopyridine with benzaldehyde or naphthaldehyde. As shown in Figs. S1a and S1b (Supporting information), the spectra signals of N1 and N2 displayed noticeable changes after the addition of HOCl. Compounds N1 and N2 were converted into triazole derivatives (NT1 and NT2) after being treated with HOCl, which were validated by HRMS (Figs. S2 and S3 in Supporting information). In light of these encouraging results, we further synthesized compounds N3-N6 (potential HOCl probes) through the condensation of 2-hydrazinopyridine and diverse aldehyde fluorophores, including coumarin (N3-N5) and xanthene (N6) scaffolds. Furthermore, we obtained the crystal structure of compound N6 (Table S1 in Supporting information). Detailed experimental procedures and characterization of compounds N1-N6 and compounds NT1-NT6 can be found in the supporting information. The spectral properties of N3-N6 before and after the addition of HOCl were investigated in a physiological buffer solution (Figs. S1c-f in Supporting information). As expected, HRMS results show that compounds N3-N6 were also converted into the corresponding triazole derivatives (NT3-NT6) after treatment with HOCl (Figs. S4–S7 in Supporting information). Moreover, taking the N6 as an example, we measured the spectra of NT6 and the reaction product of N6 with HOCl. The emission and absorption wavelengths of NT6 are consistent with that of N6 treated with HOCl, demonstrating that NT6 was the product of N6 with HOCl (Fig. S8 in Supporting information). In accordance with the optical spectrum, 1H NMR characterization data indicate that the reaction of N6 with HOCl results in the disappearance of the signal at 11.16 ppm (=N-NH- proton of N6), and the 1H NMR spectrum is well matched with that of NT6 (Fig. S9 in Supporting information). Taken together, all these results further support our hypothesis that the sensing event proceeds through HOCl-promoted triazolopyridine formation.
|
Download:
|
| Scheme 1. (a) Previous work. I2-promoted triazolopyridine formation strategy. (b) This work. Our new HOCl-promoted triazolopyridine formation strategy. (c) Synthetic route for compounds N1-N6. | |
A plausible reaction mechanism for HOCl-promoted triazolopyridine formation is shown in Scheme 1b. Taking the formation of N6 as an example, chlorination occurs on the C=N bond of N6 using Cl+ from HOCl. Then, the chloro-substituted carbon atom is then attacked by the pyridine nitrogen to produce the 3H-1, 2, 4-triazolo [4, 3-a]pyridin-4-ium intermediate through a 5-exo-tet cyclization, in which a new C—N bond is formed. Finally, the triazolopyridine framework is formed by deprotonation and rearomatization.
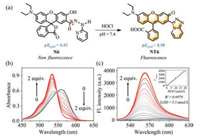
Among the compounds we designed, N6 is the most promising compound for detecting HOCl. After treatment with HOCl, N6 was transformed into triazolopyridine derivative NT6 (Fig. 1a), resulting in the generation of an intense golden fluorescence and 159-fold enhancement in fluorescence intensity. The pKcycl value of N6 was calculated to be 4.43 (Fig. S10a in Supporting information), meaning that N6 was mainly present in the unconjugated spirolactone form without fluorescence at pH 7.4. The C=N isomerization-induced fluorescence quenching is the other reason why N6 is not fluorescent causing negligible background fluorescence. On the other hand, the pKcycl of NT6 was calculated to be 8.98 (Fig. S10b in Supporting information), indicating that NT6 would mainly exist in the ring-opened π-conjugated form with strong fluorescence at pH 7.4. Therefore, the N6 displayed a rapid response (45 s), high selectivity towards HOCl over other ROS and RNS as well as sensitive detection of HOCl with a detection limit of 5.3 nmol/L. Remarkably, N6 was successfully used to visualize HOCl in living cells and zebrafish.
|
Download:
|
| Fig. 1. (a) Recognition strategy of N6 towards HOCl. (b) UV–vis absorption spectra of N6 (10 μmol/L) upon treatment with HOCl (0-2 equiv.) in pH 7.4 PBS buffer solution. (c) Fluorescence emission spectra changes of N6 (10 μmol/L) upon treatment with HOCl (0-2 equiv.) in pH 7.4 PBS buffer solution. Inset: Linear correlation of N6 towards the concentrations of HOCl. λex = 505 nm, λem = 563 nm, slit: 2.5 nm/5 nm. | |
The pH-dependent fluorescence changes of N6 towards HOCl were then evaluated. Fig. S11 (Supporting information) shows that N6 was very stabled and was able to respond to HOCl over a broad pH range from 5.5–11.0. In order to facilitate biological applications, a pH of 7.4 was selected for examining the spectral properties of probe N6. Therefore, we titrated HOCl with probe N6 in PBS buffer solution (pH 7.4) and the process was monitored by UV–vis absorption. As shown in Fig. 1b, the maximum absorption peak of probe N6 (10 μmol/L) was at 562 nm (ε = 61, 900 L mol-1 cm-1). However, with successive additions of HOCl (0–2.0 equiv.) to the solution of N6, the absorption peak at 562 nm reduced gradually and synchronously a new absorption peak emerged at 534 nm (ε = 92, 600 L mol-1 cm-1) with an isosbestic point at 548 nm. These results indicated the formation of a new product after the addition of HOCl into a solution of N6. Fluorescence titrations were then conducted in PBS buffer solution (pH 7.4) (Fig. 1c). N6 (Φf = 0.01) had minimal fluorescence, while the addition of HOCl into the N6 solution led to a 159-fold increase in fluorescence intensity at 563 nm, which was interpreted as the formation of NT6 (Φf = 0.30). Moreover, the fluorescence intensity at 563 nm displayed an excellent linear relationship (R2 = 0.9979) with HOCl concentrations ranging from 0 to 18 μmol/L. The detection limit of N6 for HOCl was calculated to be 5.3 nmol/L (Fig. 1c). All these results clearly indicate that N6 can detect HOCl quantitatively.
The time-dependence of the fluorescence intensity at 563 nm of probe N6 in the absence and presence of HOCl was also evaluated. As shown in Fig. S12 (Supporting information), with the addition of HOCl to a solution of N6, the fluorescence intensity at 563 nm gradually enhanced with increasing reaction time and reached a plateau within 45 s. The reaction rate of probe N6 with HOCl is very fast, which is crucial for real-time detection of HOCl in vivo.
To assess the selectivity of N6 towards HOCl, we investigated the fluorescence spectra of probe N6 towards various reactive nitrogen species (RNS), reactive oxygen species (ROS), common anions and biothiols. As shown in Fig. 2a, the fluorescence intensity at 563 nm was enhanced remarkably after the addition of HOCl, with an obvious fluorescence change (Fig. 2a inset), while other biologically relevant substances did not trigger any significant changes under similar conditions. Simultaneously, we investigated the absorption spectra of N6 towards various analytes. In Fig. 2b, only HOCl caused a distinct change of the absorption spectra, whereas other analytes gave rise to negligible changes. In addition, a competitive binding assay was performed by adding HOCl to the mixed solution of N6 and other species (Fig. S13 in Supporting information). The competitive experiments indicate that other analytes did not interfere with the detection of HOCl by N6. These results clearly indicate that N6 has excellent selectivity for HOCl over other analytes and can be used for the accurate detection of HOCl in complex systems.

|
Download:
|
| Fig. 2. (a) Fluorescent response of N6 (10 μmol/L) in the presence of various analytes (2 equiv.) in PBS buffer solution (20 mmol/L, pH 7.4, containing 20% EtOH). Inset: visual fluorescence color under a handheld 365 nm UV lamp. (b) UV–vis absorbance spectra of probe N6 (10 μmol/L) in the presence of various analytes (2 equiv.) in PBS buffer solution (20 mmol/L, containing 20% EtOH).1: Blank, 2: HS-, 3: F-, 4: Cl-, 5: Br-, 6: I-, 7: S2O32-, 8: AcO-, 9: SO42-, 10: NO2-, 11: NO3-, 12: CN-, 13: SCN-, 14: PO43-, 15: H2PO4-, 16: HPO42-, 17: HCO3-, 18: HSO4-, 19: HSO3-, 20: SO32-, 21: CO32-, 22: Cys, 23: Hcy, 24: GSH, 25: H2O2, 26: *O2-, 27: *OH, 28: 1O2, 29: NO*, 30: TBHP, 31: TBO*, 32: ONOO-, 33: HOCl. λex = 505 nm, λem = 563 nm, slit: 2.5 nm/5 nm. | |
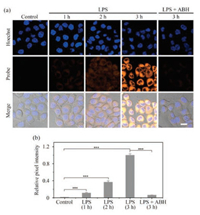
Inspired by the excellent sensing performance of N6 towards HOCl in vitro, we postulated that N6 could be used for imaging HOCl in living cells. Prior to cellular imaging tests, the cytotoxicity of N6 was evaluated using a methyl thiazolyl tetrazolium (MTT) assay in HepG2 cells. It was clear from those tests that probe N6 has low cytotoxicity. (Fig. S14 in Supporting information). Therefore, probe N6 was used to detect the level of exogenous HOCl in HepG2 cells. As shown in Fig. S15 (Supporting information), cells incubated with only probe N6 were almost non-fluorescent. After these cells were treated with 1 μmol/L, 5 μmol/L and 10 μmol/L HOCl, distinct fluorescence was observed in the yellow channel and the fluorescence intensity of the cells enhanced following the increase of HOCl concentration. Meanwhile, we also explored the ability of N6 to visualize endogenous HOCl by Bacterial endotoxin lipopolysaccharide (LPS) in HepG2 cells. LPS can stimulate cells to release ROS, such as HOCl [16]. As shown in Fig. 3, no fluorescence was observed in HepG2 cells incubated with only probe N6. When N6-stained cells were incubated with LPS, the fluorescence signal in the yellow channel became brighter with increasing incubation time from 0 to 3 h. In contrast, 4-aminobenzoic hydrazide (ABH), a well-known MPO inhibitor, can reduce the concentration of endogenous HOCl [15]. As expected, the intracellular fluorescence intensity is clearly decreased in the presence of ABH. These results indicate that probe N6 is cell membrane permeable and capable of imaging exogenous and endogenous HOCl in living cells without interference from background signals.
|
Download:
|
| Fig. 3. (a) Confocal fluorescent images of N6 in HepG2 cells under different conditions. HepG2 cells pretreated with LPS for 0 h (first column), 1 h (second column), 2 h (third column) and 3 h (fourth column), and then stained with N6 (5 μmol/L, 5 min) and Hoechst (10 μg/mL, 20 min); Cells (fifth column) were stimulated with LPS (5 μg/mL) and ABH (250 μmol/L) for 3 h, and then incubated with N6 (5 μmol/L, 5 min) and Hoechst (10 μg/mL, 20 min). (b) Normalized intensity of the fluorescence images of (a). The results are presented as the mean ± SD (n = 3). ***P < 0.001, two-sided Student's t-test. (Hoechst, λex = 405 nm, λem = 425-475 nm; Probe, λex = 488 nm, λem = 500-550 nm; scale bar = 20 μm). | |
Encouraged by the results for cell imaging, we explored the capability of N6 to image HOCl in zebrafish. As shown in Fig. S16 (Supporting information), zebrafish incubated with N6 (50 μmol/L) for 30 min displayed low fluorescence in the yellow channel. Then zebrafish stained by N6 were incubated with different amounts of HOCl (0, 10, 50 and 100 μmol/L) for 30 min and significant yellow fluorescence was observed. To assess the performance of N6 in imaging the endogenous HOCl in zebrafish, we further pre-incubated zebrafish with LPS and then with probe N6. As shown in Fig. 4, the fluorescence intensity of the zebrafish stimulated by LPS exhibited a time-dependent increase with prolonging the incubation time (0-3 h) and we observed that the endogenous HOCl induced by LPS is mainly accumulated in yolk, intestine, kidney and liver of zebrafish (Fig. 4c). Hence, these results indicate that probe N6 can detect exogenous and endogenous HOCl in vivo

|
Download:
|
| Fig. 4. (a) Representative images from the four groups of zebrafish. Zebrafish pretreated with LPS for 0 h, 1 h, 2 h and 3 h (from top to bottom), and then stained with N6. (b) Normalized intensity of the fluorescence images of (a). The results are presented as the mean ± SD (n = 3). ***P < 0.001, two-sided Student's t-test. (c) Zoom image of the area indicated with red square (regions: 1, yolk; 2, intestine; 3, kidney; 4, liver). λex = 488 nm, λem = 500–550 nm, scale bar = 500 mm. | |
In conclusion, we report on a new strategy in which a 2-pyridylhydrazone moiety is selectively oxidized by HOCl to 1, 2, 4-triazolo[4, 3-a]pyridine. Featured by mild reaction conditions, triazolopyridines NT1-NT6 have been efficiently synthesized from various 2-pyridylhydrazones. We introduce 2-pyridylhydrazone as a new recognition receptor for HOCl and in particular N6 with a rhodol skeleton has been developed as a highly selective fluorescent probe for the detection of HOCl in vitro and in vivo. This probe displayed a 159-fold rapid (45 s) fluorescence turn-on response with low limit of detection (5.3 nmol/L) for HOCl. Furthermore, probe N6 was used to image HOCl in living cells and zebrafish respectively with promising results, clearly demonstrating its effectiveness in biological systems. Our present HOCl detection system based on HOCl-promoted triazolopyridine formation is unique and offers a general strategy to design new HOCl fluorescent probes.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21977082, 21472148 and 21807088), Open Funding Project of the State Key Laboratory of Bioreactor Engineering (No. 2018OPEN12), Special Foundation of the Education Committee of Shaanxi Province (No. 18JK0702), Technology Plan Project of Xi'an (Nos. 201805040YD18CG24 and GXYD18.1) and Academic Backbone of Northwest University Outstanding Youth Support Program. Tony D. James wishes to thank the Royal Society for a Wolfson Research Merit Award.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.020.
| [1] |
J. Zielonka, J. Joseph, A. Sikora, et al., Chem. Rev. 117 (2017) 10043-10120. DOI:10.1021/acs.chemrev.7b00042 |
| [2] |
T. Nybo, S. Dieterich, L.F. Gamon, et al., Redox Biol. 20 (2019) 496-513. DOI:10.1016/j.redox.2018.10.022 |
| [3] |
T.H.D. Araujo, S.S. Okada, E.E.B. Ghosn, et al., Cell. Immunol. 281 (2013) 27-30. DOI:10.1016/j.cellimm.2013.01.002 |
| [4] |
L. Wu, I.C. Wu, C.C. DuFort, et al., J. Am. Chem. Soc. 139 (2017) 6911-6918. DOI:10.1021/jacs.7b01545 |
| [5] |
Z. Mao, M. Ye, W. Hu, et al., Chem. Sci. 9 (2018) 6035-6040. DOI:10.1039/C8SC01697F |
| [6] |
J. Luo, K. Mills, S.L. Cessie, R. Noordam, D.V. Heemst, Ageing Res. Rev. 57 (2020) 100982. DOI:10.1016/j.arr.2019.100982 |
| [7] |
D.I. Pattison, M.J. Davies, Biochemistry 45 (2006) 8152-8162. DOI:10.1021/bi060348s |
| [8] |
J. Kay, E. Thadhani, L. Samson, B. Engelward, DNA Repair 83 (2019) 102673. DOI:10.1016/j.dnarep.2019.102673 |
| [9] |
P. Palladino, F. Torrini, S. Scarano, M. Minunni, J. Pharmaceut. Biomed. 179 (2020) 113016. DOI:10.1016/j.jpba.2019.113016 |
| [10] |
D. Shi, S. Chen, B. Dong, et al., Chem. Sci. 10 (2019) 3715-3722. DOI:10.1039/C9SC00180H |
| [11] |
H. Xiong, L. He, Y. Zhang, et al., Chin. Chem. Lett. 30 (2019) 1075-1077. DOI:10.1016/j.cclet.2019.02.008 |
| [12] |
Y. Chen, T. Wei, Z. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1957-1960. DOI:10.1016/j.cclet.2017.05.010 |
| [13] |
J. Lv, F. Wang, T. Wei, X. Chen, Ind. Eng. Chem. Res. 56 (2017) 3757-3764. DOI:10.1021/acs.iecr.7b00381 |
| [14] |
Y.L. Pak, S.J. Park, G. Song, et al., Anal. Chem. 90 (2018) 12937-12943. DOI:10.1021/acs.analchem.8b03565 |
| [15] |
P. Wei, L. Liu, Y. Wen, et al., Angew. Chem. Int. Ed. 58 (2019) 4547-4551. DOI:10.1002/anie.201813648 |
| [16] |
X. Zhang, W. Zhao, B. Li, et al., Chem. Sci. 9 (2018) 8207-8212. DOI:10.1039/C8SC03226B |
| [17] |
X. Chen, F. Wang, J.Y. Hyun, et al., Chem. Soc. Rev. 45 (2016) 2976-3016. DOI:10.1039/C6CS00192K |
| [18] |
Y.L. Pak, S.J. Park, D. Wu, et al., Angew. Chem. Int. Ed. 57 (2018) 1567-1571. DOI:10.1002/anie.201711188 |
| [19] |
J. Lv, Y. Chen, F. Wang, et al., Dyes Pigments 148 (2018) 353-358. DOI:10.1016/j.dyepig.2017.09.037 |
| [20] |
X. Cheng, H. Jia, T. Long, et al., Chem. Commun. 47 (2011) 11978-11980. DOI:10.1039/c1cc15214a |
| [21] |
B. Wang, D. Chen, S. Kambam, et al., Dyes Pigments 120 (2015) 22-29. DOI:10.1016/j.dyepig.2015.03.022 |
| [22] |
W.L. Wu, Z.M. Zhao, X. Dai, L. Su, B.X. Zhao, Sens. Actuators B 232 (2016) 390-395. DOI:10.1016/j.snb.2016.03.155 |
| [23] |
Y. Zhang, L. Ma, C. Tang, et al., J. Mater. Chem. B 6 (2018) 725-731. DOI:10.1039/C7TB02862H |
| [24] |
E. Li, Z. Hu, L. Song, W. Yu, J. Chang, Chem. Eur. J. 22 (2016) 11022-11027. DOI:10.1002/chem.201601744 |
 2020, Vol. 31
2020, Vol. 31 

