b Beijing National Laboratory for Molecular Sciences, Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
c University of Chinese Academy of Sciences, Beijing 100049, China;
d Beijing Key Laboratory for Optical Materials and Photonic Devices, Department of Chemistry, Capital Normal University, Beijing 100048, China;
e Collaborative Innovation Center of Chemical Science and Engineering (Tianjin) & Department of Chemistry, School of Science, Tianjin University, Tianjin 300072, China
Potential-of-hydrogen (pH) sensors are devices that respond specifically to acids or bases and are capable of detecting the concentration of hydrogen ions in substances. It should be mentioned that pH sensors have an irreplaceable role in the field of environmental protection and biological medicine [1, 2]. As a common pH sensor, pH test paper has the ability to accurately and sensitively detect large range of hydrogen ion concentration of substances. This ability is based on methyl red, bromocresol green, and thymol blue, but these three acid-sensitive materials are wasted because they cannot be reused. With the development of organic optoelectronic field, numbers of new multifunctional organic semiconductor materials have been continuously synthesized to use in various fields like information and communication technology, biomedicine and computing [3-8]. Some semicon-ducting materials have been applied to sensor devices, which are widely used to test different parameters, such as pressure, hydrogen-ion concentration, metal-ion concentration and others [9-13]. Moreover, the materials with stimulus-response-luminescence properties demonstrate great potential for application in pH sensors due to their intuitive and rapid response with the changes of pH. Tian and co-workers have reported a series of molecules, such as 1, 1'-(2, 5-distyryl1, 4-phenylene)dipiperidine (DPD), 9, 10-bis(2-(pyridine-3-yl)vinyl)anthracene (BP3VA) and 9, 10-bis((E)-2-(pyridine-4-yl)vinyl)anthracene (BP4VA), which were constructed by pyridine [14-16]. Because of the presence of acid, all these materials have abilities of fluorescent color changing, and some molecules even have electrical properties. BP3VA solid can be discolored in the acidic gas and reused by fumigation under triethylamine vapor. DPD solution implements accurate fluorescent colors changes from pH 2 to pH 0. BP4VA regulates the growth of two different crystal phases by changing the types of acid, which provides an important reference for studying the mechanism of acid reaction discoloration. However, these materials cannot be accurately used in large range of pH value or cannot be reusable, so there is still room for improvement.
Molecular design is a direct way to adjust the properties of organic stimuli-responsive luminescent materials [17]. In order to maintain the response of acid stimulation and have luminescence performance, quinoline group was introduced into the molecule [18]. Based on the extensive applications of quinoline in fluorescent materials [19, 20], it is also possible to regulate the optical properties. Protonation of nitrogen-containing groups under the action of acids leads to different fluorescent colors due to changed stacking patterns. Nitrogen atoms are kept at both ends of the molecule so that the protonated material can return to its original state under the action of a base. Therefore, 6-quinoline is used to reduce steric hindrance. Based on above-mentioned considerations, herein, we design and synthesize a new acidresponsive luminescent material, 1, 4-di(quinoline-6-yl)buta-1, 3-diyne (DQBD). As expected, upon adding hydrochloric acid, an obvious change of fluorescent characteristics for DQBD is observed due to the protonation effect, which results in different molecular packing and optical properties. Single crystal data of DQBD and protonated DQBD have well confirmed this point. Moreover, the acid-responsivity can be reversible with ability of multiple cycles by adjusting the repeated pH of sodium hydroxide and hydrochloric acid, suggesting the great potential of DQBD for pH sensors.
DQBD was synthesized by one-step reaction with 80% yield. The synthesized route and the characterized data are summarized in Scheme S1 (Supporting information). The protonation reaction process can be described by a chemical equation as shown in Fig. 1a. Part of DQBD is protonated with the decrease of pH value, which is ascribed as the reaction of unilateral quinoline, while at higher acid concentration, both quinoline sides could be protonated leading to the obvious change of its florescence color. Ultraviolet-visible (UV–vis) absorption spectra in Fig. 1b and fluorescence spectra (FL) in Fig. 1c were characterized to investigate the optical properties of DQBD solution at different pH values in the Britton-Robinson buffer solutions [21, 22]. As shown in Fig. 1b, a new broad peak located at around 380 nm appeared for DQBD solution with low pH value due to the protonation effect. More interestingly, with the change of pH values, the fluorescence spectrum of DQBD solution demonstrates obvious change with the new appearance of emission peak at 495 nm for the formation of protonated DQBD species along with the decrease of the initial emission peak at 366 nm for DQBD compound. Fig. S1 (Supporting information) shows the CIE 1931 (x, y) chromaticity diagram of the DQBD solutions under different pH values and Fig. 1d shows the fluorescence images of DQBD solution under different pH values in buffer solutions taken under the UV light illumination. Fig. 1e is the curve of fluorescence intensity ratios of the molecular forms (I366/I495) versus pH values, demonstrating a liner relationship in a wide pH range of 1.8–9.0 and suggesting its wide application range for pH detection. Based on the relationship between fluorescence intensity and pH values (Fig. S2 in Supporting information) and the HendersonHasselbach-type mass action equation: log(Fmax-F)/(F-Fmin) = pH - pKa where Fmax, Fmin, and F represent the maximum, minimum, and observed fluorescence intensity at a given pH value, respectively [23, 24], the calculated pKa value for DQBD is at around 2.1 ±0.1.

|
Download:
|
| Fig. 1. (a) Reaction process of DQBD protonation. (b) UV–vis absorption and (c) FL spectra of DQBD in Britton-Robinson buffer solutions under different pH values. (d) Fluorescence images of DQBD buffer solution under different pH values. (e) The fluorescence intensities of the molecular forms (I365/I495) versus the pH values. | |
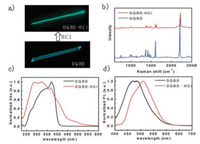
The DQBD-hydrochloric acid (DQBD-HCl) and DQBD crystals were made by solvent evaporation approach. The different crystals excited by wavelength of 365 nm are demonstrated in Fig. 2a, which directly and concretely reflects the fluorescent color change between single crystals of DQBD and DQBD-HCl. Raman spectra in Fig. 2b shows the peak of DQBD-HCl is obviously shifted compared with DQBD and that means bond environment have changed. Compared with DQBD, the UV–vis absorption of DQBD-HCl in Fig. 2c shows obvious change, which reveals the change of band gap [16]. FL of DQBD-HCl crystals have a red shift compared with DQBD crystals (Fig. 2d). Red-shifted and broader emission spectra of DQBD solution with different concentration and crystal (Fig. S3 in Supporting information) suggested the aggregation and excimer formation of DQBD since DQBD has a relative flat structure with a small torsion angle of 4.32° [25, 26].
|
Download:
|
| Fig. 2. (a) Florescence image of individual DQBD and DQBD-HCl crystal. (b) Raman spectrum of DQBD and DQBD-HCl. (c) UV–vis absorption and (d) FL spectra of DQBD/DQBD-HCl crystals. | |
Large single crystals of DQBD and DQBD-HCl crystals were obtained from the solvent evaporation approach. Compared with DQBD crystal (Fig. 3a), DQBD-HCl crystal (Fig. 3b) has more hydrogen and chlorine atoms around it. And we get the distinct stacking patterns of two crystals by following X-ray structural analysis. These crystals belong to the monoclinic system. DQBD crystal pack in a sandwich herringbone fashion clustered by along to b axis in each column (Fig. 3c). Each molecule is connected to the neighboring molecules by weak C-H ⋯ C interactions as shown in Fig. S4a (Supporting information). Molecular center distance between Quinoline group and neighboring DQBD is D1= 6.083 Å. DQBD neighboring molecular angle: θ = 75.93° (Fig. 3e). Angle of two quinolines in the same DQBD molecule is 4.42° (Fig. S4b in Supporting information). As a result, DQBD along b-axis π-π overlap by edge-to-face stacking [27]. Stacking mode of DQBD is different after the protonation of quinoline moieties with chloridion. The DQBD-HCl molecules pack in an absolute uniaxial orientation (Fig. 3d). Clustered along the c axis in each column, the molecules are through the quinoline ring of the π-π stacking interaction. Each molecule is connected to the neighboring molecules by X-H ⋯ Cl (X=C, N) as shown in Fig. S5 (Supporting information). After the protonation, due to strong hydrogen bonding, the molecular stacking structure is changed. Molecular center distance of DQBD-HCl: D2= 5.153 Å. And the interplane distance of the neighboring molecular is L = 3.394 Å. Two quinoline groups of DQBD-HCl are in the same plane (Fig. S5b in Supporting information). So that, DQBD-HCl along c-axis exhibits a certain π-π overlap by offset stacking. A certain deviation between the two conjugate planes avoids the electrostatic repulsion of the torus in the offset stacking which cause the redshift of fluorescence spectra of DQBD [28-30].

|
Download:
|
| Fig. 3. The molecular configuration of one DQBD (a) and one DQBD-HCl (b) molecule in single crystals. (c) Molecular stacking of DQBD crystals viewed along the a-axis. (d) Molecule stacking of DQBD-HCl crystals viewed along b-axis. Neighboring molecular stacking of (e) DQBD and (f) DQBD-HCl in crystals. Neighboring quinoline center distance of DQBD: D1= 6.083 Å. Molecular center distance of DQBD-HCl: D2= 5.153 Å; DQBD neighboring molecular angle: θ = 75.93°; interplane distance of DQBD-HCl: L = 3.394 Å. | |
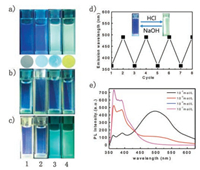
To explore the ability of repeated discoloration for DQBD, we first investigated the fluorescence characteristic of DQBD solution (Group 1, Fig. 4a) with addition of difference concentrations of 10-7 mol/L (1), 10-4 mol/L (2), 10-3 mol/L (3) and 10-2 mol/L (4) of HCl. As anticipated from the above results, gradual fluorescence colors were observed for these solutions due to the different pH values. In this case, when we added sodium hydroxide into these solutions to adjust its neutral property, similar fluorescence color was observed under the neutral conditions (Group 2, Fig. 4b). Further adding HCl into the Group 2 solutions, reversible florescence colors were observed again that was similar to that of Group 1, suggesting the its good reversibility for pH sensor applications. In addition, by absorbing DQBD onto thin-layer silica gel plates and then dropping HCl with different concentrations, similar florescence color changes with that in solution were also observed (Fig. 4a, below), suggesting its potential use for pH paper. It should be stated that it is difficult for DQBD films to achieve quick response for the detection of HCl gas because of the inadequate protonation effect within a relatively short time. Fig. 4d shows the reversable changes for fluorescence intensity under different pH values which demonstrates good stable and reversibility. Fig. 4e shows the corresponding FL spectra of DQBD solutions in accordance with that shown in Fig. 4a. Although different acids were substituted, the spectral morphology was similar to that of the buffer solution. As shown in Fig. S7 (Supporting information), FL spectra of four samples of Fig. 4d (Cycle 1, Cycle 2, Cycle 7, Cycle 8) almost coincide at the same acid – base properties. The results show that the emission wavelength of the reused solution is basically the same under the same concentration of HCl. In summary, after eight times of acid - base changing, DQBD can still be used with high accuracy, which promises a potential application of DQBD on reusable and accurate pH sensors. In addition, the pH testing ability of DQBD for other acids such as acetic acid, trifluoroacetic, nitric acid and sulfuric acid has also been investigated in our experiment. As shown in Fig. S6 (Supporting information), obvious emission changes could be easily observed under a certain concentration, suggesting its wide applications in various fields.
|
Download:
|
| Fig. 4. (a) Fluorescent images of DQBD in different concentrations of HCl solutions (Group 1), below is the fluorescence images of DQBD films after adding different concentration of HCl solution on top. (b) Fluorescent image of Group 1 is adjusted to neutral by NaOH. (Group 2) (c) Fluorescent pictures of solution in Group 2 adjusted to previous concentration of HCl. (Group 3) (d) Primary emission wavelengths of DQBD solution by repeated adjusting with HCl and NaOH solution for 8 times. (e) FL spectra of the solution in (a). | |
In this article, a new acid-responsive luminescent organic material, DQBD is designed and synthesized. Excellent pH sensor property including obvious fluorescence change for sensor and reversible response ability is characterized for DQBD compound due to the controllable protonation effect on the functional quinoline groups. Single crystal data of DQBD and DQBD-HCl definitely demonstrate the change of molecular packing after protonation, which leads to optical property changes for application in pH sensor. In addition, it should be stated that another interesting character for DQBD-HCl is that the molecular packing in this state demonstrates a slipped packing with the C1-C4 distance of 3.5 Å with an angle of 45° between two neighboring diacetylene, suggesting its ability for preparation of topochemical polymerized polydiacetylene single crystals, which has been proved to be good change-transporting materials for optoelectronic devices and fundamental studies [31-34]. In-depth studies are on going in our experiment
Declaration of competing interestWe declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled
AcknowledgmentsThe authors thank very much for the valuable discussion of Prof. Zhen Li from Wuhan University and the valuable suggestion of associate professor Yanyan Fu from Shanghai institute of Microsystems, CAS. The authors also acknowledge financial support from the Ministry of Science and Technology of China (Nos. 2017YFA0204503, 2016YFB0401100), the National Natural Science Foundation of China (Nos. 21875259, 51725304, 51633006, 61890943, 91833306, 51822308, 21975263), the Strategic Priority Research Program (No. XDB12030300) of the Chinese Academy of Sciences and Beijing National Laboratory for Molecular Sciences (No. BNLMS-CXXM-202012).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.02.012.
| [1] |
El-khouri R.J., D.A. Bricarello, E.B. Watkins, et al., Nano Lett. 11 (2011) 2169-2172. DOI:10.1021/nl200832c |
| [2] |
Z.C. Zhang, Q.G. Lai, Y.A. Li, C. Xu, et al., Sci. Rep. 7 (2017) 46161. DOI:10.1038/srep46161 |
| [3] |
P.C. Gu, M.X. Hu, S. Ding, et al., Chin. Chem. Lett. 29 (2018) 1675-1680. DOI:10.1016/j.cclet.2018.03.034 |
| [4] |
X.T. Zhang, H.L. Dong, W.P. Hu, Adv. Mater 30 (2018) 1801048. DOI:10.1002/adma.201801048 |
| [5] |
Y.G. Zhen, H.L. Dong, L. Jiang, et al., Chin. Chem. Lett. 27 (2016) 1330-1338. DOI:10.1016/j.cclet.2016.06.023 |
| [6] |
H.L. Dong, H.F. Zhu, Q. Meng, et al., Chem. Soc. Rev. 41 (2012) 1754-1808. DOI:10.1039/C1CS15205J |
| [7] |
M.X. Hu, J.Y. Liu, Q. Zhao, et al., Sci. China-Mater. 62 (2019) 729-735. DOI:10.1007/s40843-018-9369-5 |
| [8] |
X. Qin, H.L. Dong, W.P. Hu, Sci. China-Mater. 58 (2015) 186-191. DOI:10.1007/s40843-015-0035-4 |
| [9] |
Y. Li, X. Wang, C.Y. Xing, X.R. Zhang, et al., Chin. Chem. Lett. 30 (2019) 1440-1444. DOI:10.1016/j.cclet.2019.03.014 |
| [10] |
D. Yi, Y.P. Wu, C.Y. Chen, et al., Chin. Chem. Lett. 30 (2019) 1059-1062. DOI:10.1016/j.cclet.2019.01.013 |
| [11] |
S.J. Yoon, J.W. Chung, J. Gierschner, et al., J. Am. Chem. Soc. 132 (2010) 13675-13683. DOI:10.1021/ja1044665 |
| [12] |
Y.Y. Fu, H.X. Li, W.P. Hu, Eur. J. Org. Chem. 2007 (2007) 2459-2463. DOI:10.1002/ejoc.200601086 |
| [13] |
J.N. Wilson, U.H.F. Bunz, J. Am. Chem. Soc. 127 (2005) 4124-4125. DOI:10.1021/ja050017n |
| [14] |
J.B. Zhang, J.L. Chen, B. Xu, et al., Chem. Commun. 49 (2013) 3878-3880. DOI:10.1039/c3cc41171k |
| [15] |
J.L. Chen, S.Q. Ma, J.B. Zhang, et al., J. Phys. Chem. Lett. 5 (2014) 2781-2784. DOI:10.1021/jz501383d |
| [16] |
S.Q. Ma, J.B. Zhang, Y.J. Liu, et al., J. Phys. Chem. Lett. 8 (2017) 3068-3072. DOI:10.1021/acs.jpclett.7b01454 |
| [17] |
D. Liu, C.G. Li, S.J. Niu, et al., J. Mater. Chem. C 7 (2019) 5925-5930. DOI:10.1039/C9TC01321K |
| [18] |
Z.J. Ni, H.L. Dong, H.L. Wang, et al., Adv. Mater. 30 (2018) 1704843. DOI:10.1002/adma.201704843 |
| [19] |
J.V. Jun, E.J. Petersson, D.M. Chenoweth, J. Am. Chem. Soc. 140 (2018) 9486-9493. DOI:10.1021/jacs.8b03738 |
| [20] |
C.Y. Fu, T. Kobayashi, N.X. Wang, et al., J. Am. Chem. Soc. 140 (2018) 11058-11066. DOI:10.1021/jacs.8b05814 |
| [21] |
H.T.S. Britton, R.A. Robinson, J. Chem. Soc. (1931) 1456-1462. |
| [22] |
C.D. Dou, L. Han, S.S. Zhao, et al., J. Phys. Chem. Lett. 2 (2011) 666-670. DOI:10.1021/jz200140c |
| [23] |
J. Mandal, P. Ghorai, P. Brandão, New J. Chem. 42 (2018) 19818-19826. DOI:10.1039/C8NJ04753G |
| [24] |
L.X. Cao, X.Y. Li, S.Q. Wang, et al., Chem. Commun. 50 (2014) 8787-8790. DOI:10.1039/C4CC03716B |
| [25] |
J.L. Banal, J.M. White, T.W. Lam, et al., Adv. Energy Mater. (2015) 1500818. |
| [26] |
J.Z. Fan, L.L. Lin, C.K. Wang, Phys. Chem. Chem. Phys. 19 (2017) 30147-30156. DOI:10.1039/C7CP05451C |
| [27] |
W.B. Jennings, B.M. Farrell, J.F. Malone, Acc. Chem. Res. 34 (2001) 885-894. DOI:10.1021/ar0100475 |
| [28] |
M.D. Curtis, J. Cao, J.W. Kampf, J. Am. Chem. Soc. 126 (2003) 4318-4328. |
| [29] |
D.N. Beratan, J.N. Onuchic, J.N. Betts, et al., J. Am. Chem. Soc. 112 (1990) 7915-7921. DOI:10.1021/ja00178a011 |
| [30] |
H.Y. Zhang, Z.L. Zhang, K.Q. Ye, et al., Adv. Mater. 18 (2006) 2369-2372. DOI:10.1002/adma.200600704 |
| [31] |
Q.Y. Li, Y.F. Yao, G. Qiu, et al., Chin. Sci. Bull. 61 (2016) 2688-2706. DOI:10.1360/N972016-00462 |
| [32] |
Q.Q. Yan, Y.F. Yao, H.L. Dong, et al., Sci. Sin. Chim. 46 (2016) 1007-1022. DOI:10.1360/N032016-00065 |
| [33] |
Y.F. Yao, H.L. Dong, F. Liu, et al., Adv. Mater. 29 (2017) 1701251. DOI:10.1002/adma.201701251 |
| [34] |
H.L. Dong, W.P. Hu, Acta Polym. Sin. 8 (2017) 1246-1260. |
 2020, Vol. 31
2020, Vol. 31 

