b School of Energy and Environmental Engineering, Hebei University of Technology, Tianjin 300401, China
Antibiotics are widely used and often misused in human medicine and stockbreeding operations [1], which have led to the emergence and proliferation of antibiotic resistance genes (ARGs) in the environment [2]. What is worse is that ARGs could permanently exist even in the absence of antibiotic selective pressure [3]. ARGs also have the potential to be widely distributed to various environmental compartments [4, 5]. To date, diverse kinds of ARGs have been detected in surface water [6], groundwater [7] and even in drinking water [8]. Aquatic ecosystems are recognized as reservoirs for ARGs and vehicle of antibiotic resistance. ARGs in water may pose the ubiquitous propagation of antibiotic resistance [9] and serious risks to both human health and ecosystem [10]. As one of the most critical human health challenges, ARGs have gained increasing attention all around the world [11]. Therefore, it is necessary to remove ARGs from the water environment (Text S1 in Supporting information).
Two kinds of ARGs are present in water, namely intracellular ARGs (iARGs) and extracellular ARGs (eARGs) [12]. iARGs are harbored via antibiotic resistance bacteria (ARB), and eARGs originate from the secretionofliveARB [13].Afterentering the environment, eARGsare ready to be assimilated by indigenous competent bacteria via transformation [14]. When the bacteria harboring iARGs die, iARGs can be released to the environment again by the lysis of dead cells [15, 16] and still staybioavailable [17]. eARGs in the environment are relatively stable and often persisting for months [18]. The circulation of eARGs contributes to the widespread of antibiotic resistance, which emphasizes the demand for elimination of eARGs. However, a very limited number of attempts have been made to solve this problem. McKinney and Pruden [11] demonstrated that ultraviolet disinfection held weak potential to damage ARGs in water. Yuan et al. [19] demonstrated that the reductions of erythromycin and tetracycline resistance genes by chlorination were approximate 0.42 log and 0.10 log. Guo et al. [20] discovered that low dose of chlorine (40 mg Cl min/L) even promote horizontal gene transfer and spread of antibiotic resistance. Hence, the aforementioned methodologies have some limitations and it is urgent to explore more effective removal methods.
Adsorption technique has been extensively applied in wastewater treatment due to its ease of operation, high efficiency and economical cost [21]. The choice of adsorbent is an essential step for adsorption process. Graphitic carbon nitride (g-C3N4) with chemical stability and nontoxic property has captured the concern of numerous researchers [22]. Compared with common carbon materials (graphene, activated carbon, etc.), g-C3N4 has the advantages of high stability, low synthesis cost, high selectivity, easy regeneration and high recyclability, which well overcomes the typical defects of common environmental protection materials, such as poor selectivity and high energy consumption regeneration [23, 24]. Meanwhile, due to its large specific surface area and abundant functional groups containing nitrogen on the surface, it can provide suitable sites for the adsorption of ARGs [25]. g-C3N4 an be easily synthesized from abundant precursors via the polymerization method [26], which is suitable for large scale production. Melamine [27], cyanamide [28], dicyandiamide [29], thiourea [30] and urea [31] are commonly used as precursors. As a nitrogen-rich and oxygen-containing compound, urea is low-cost and readily available. Nowadays, g-C3N4 has played important roles in degradation of organic pollutants [32], adsorption of heavy metal ions [33], photocatalytic reduction of CO2 [34] and NO [35]. However, to the best of our knowledge, the adsorption of ARGs on g-C3N4 has never been reported. In this study, our attention is focused on two representative eARGs (ampC and ermB). β-Lactam antibiotics are the most consumed antibiotics globally [36], and the gene ampC encoding resistance to β-lactam has been detected in wastewater, surface water, and even from drinking water films [37]. Meanwhile, the gene ermB (against macrolides) is one of the most prevalent erythromycin resistance genes in the environment [38].
The objective of this study is to examine the potential of g-C3N4 for removing eARGs from water. We employed the thermal polymerization method to synthesize g-C3N4 powders with urea as a precursor. A series of adsorption experiments were performed with the as-prepared g-C3N4. Quantitative real-time polymerase chain reaction (qPCR) was used to determine the concentrations of the target ARGs in aqueous solution. g-C3N4 powders were systematically characterized by scanning electron microscope (SEM), transmission electron microscope (TEM), X-ray diffraction (XRD), Brunauer-Emmett-Teller (BET) surface area, Fourier transform infrared (FTIR), X-ray photoelectron spectroscopy (XPS). The zeta potentials of the g-C3N4 powders and ARGs were measured and the isoelectric point (IEP) was discussed. Adsorption isotherms and kinetics were investigated to confirm the adsorption capacity of g-C3N4. We also took into consideration of two parameters (pH and temperature) in the experiment. The mechanisms that underline the adsorption process were explored and analyzed. Furthermore, we fabricated a column to remove ARGs by filtration and validated its efficiency.
Detailed information on the synthesis, characterization, sample collection, ARGs extraction and adsorption experiments of g-C3N4 is provided in the Texts S2-5 (Supporting information). SEM, TEM, XPS, XRD, FTIR, nitrogen adsorption-desorption isotherm and pore diameter distribution analysis were shown in Text S6 (Supporting information).
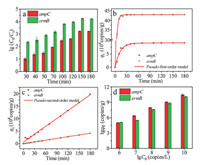
Adsorption is a physicochemical process that involves mass transfer of solute from liquid phase to the surface of adsorbent [39]. Kinetics study can provide necessary information to describe the adsorption rate of g-C3N4 and control the residual time of the whole adsorption process [40]. The adsorption kinetics were investigated by adding the as-prepared g-C3N4 powders to the solutions containing the target ARGs. Initial concentrations of ampC and ermB were examined and recorded. The time-dependent adsorption curves of ampC and ermB are depicted in Fig. 1a. It is apparent that with the passage of time, the values of lgC0/C for both ARGs continually increase. This experiment maintains a continuous and homogeneous adsorption process. There are no significant differences in the trends of two curves. The adsorption equilibrium for both ARGs can be achieved within 180 min. The lgC0/C values of two ARGs (ampC and ermB) at equilibrium are 3.2 and 4.2, indicating good efficiency of g-C3N4 as an adsorbent. Actually, the adsorption rate of ermB on g-C3N4 is higher than that of ampC, reflected by the higher initial slope of the adsorption kinetic curve, which can be attributed to the deoxyribonucleic acid (DNA) length of ermB (189 bp) is shorter than that of ampC (193 bp). In a study by Wu et al. [41], shorter DNA are adsorbed more rapidly and bind more tightly to the surface of graphene oxide. Li and Rothberg [42] have demonstrated that the adsorption rate of single-stranded DNA depend on DNA length and temperature. Subsequently, they have further confirmed that longer single-stranded DNA sequences were adsorbed on the Au nanoparticles at a much slower rate [43]. In this study, the results are in accordance with previous researches. In addition, the adsorption of ARGs on g-C3N4 may be related to its base composition [44, 45].
|
Download:
|
| Fig. 1. Adsorption kinetics of ARGs on g-C3N4 powders (a). The fitting curves of adsorption kinetics: Pseudo-first-order model (b), and pseudo-second-order model (c). The adsorption capacity (lgqe) changes with initial ion concentration (lgC0) at room temperature (d). | |
For the sake of evaluating the adsorption mechanism in depth, the pseudo-first-order and pseudo-second-order models were used to analyze the data of adsorption kinetics. The two models can be described by equations (Eqs. 1 and 2), respectively.

|
(1) |
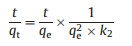
|
(2) |
where qt (copies/L) and qe (copies/g) are the amounts of ARGs adsorbed on the g-C3N4 at time t (min) and at equilibrium, respectively. k1, (min -1), k2, (copies g-1 min-1) are the rate constants of two models. The k1, k2, and qe could be calculated from the slope and intercept of the curves.
The correlation coefficient R2, is important to estimate the suitability of different equation models. The higher the value of R2, is, the more accurately the model describes the adsorption kinetics. The results are summarized in Table S2 (Supporting information). As shown in Figs. 1b and c, the pseudo-second-order model provided better fitting for all the experimental data. The correlation coefficients are higher than 0.994, indicating that in this experiment adsorption is probably dominated by a monolayer adsorption [46]. The adsorption of g-C3N4 to ARGs may be due to the triazine ring fabricating by C and N atoms which form the highly delocalized π conjugated system through sp2 hybridization, providing a possibility to fix the DNA molecule on it by π-π bond adsorption because of the π conjugated on the nucleotide [47]. In addition, the nitrogen of base ring and the exocyclic keto groups of ARGs may also be responsible for the binding of ARGs to g-C3N4 through chemical binding on amine groups and other surface groups on g-C3N4. The rate-limiting step may involve valency forces through sharing or the exchange of electrons [48]. The qe of ermB was larger than that of ampC. Their values of k2, were also in the order of ermB > ampC. It indicated that the length of DNA may affect the adsorption process.
To determine the maximum adsorption capacity, the experiments of adsorption isotherms were carried out with varied initial concentrations of ARGs at room temperature. Preliminarily tests indicated experimental time was supposed to be set longer than 180 min. As shown in Fig. 1d higher C0 values brought in higher values of qe. The adsorption capability increased with the rise of initial concentrations for both the gene ampC and ermB. Driving force generated by the pressure of concentration gradient could account for these phenomena. The interaction between the adsorbate and adsorbent can be described by two common isotherm equilibrium models, namely the Langmuir and Freundlich models. The equations of the two models can be expressed as follows (Eqs. 3 and 4):
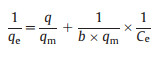
|
(3) |
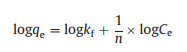
|
(4) |
In these equations, qm (copies/g) is the maximum adsorption amount; Ce (copies/L) is the equilibrium concentration; b, kf and n are the constants of two models.
The parameters of the two models were listed in Table S3 (Supporting information). The linear fitting results of Langmuir model and Freundlich model are shown in Fig. 2. The maximum adsorption capacities of ampC and ermB at 25 ℃ are 7.2 × 105 copies/g and 10.6 105 copies/g, separately. The correlation coefficients (R2, ) of Langmuir model for ampC and ermB are both above 0.97, higher than those of Freundlich model (0.60 for ampC and 0.93 for ermB). The experimental data are better simulated by the Langmuir model. In fact, the Langmuir isotherm is generally established on the assumption that the surface of the absorbent is homogenous, which means every adsorption site has equal affinity with the adsorbate. In this experiment, it means a monolayer adsorption, which is suitable for the adsorption of ampC and ermB on g-C3N4 powders. In addition, RL, namely the separation factor, is an essential characteristic of the Langmuir isotherm to predict the affinity, which could be calculated from the following equation (Eq. 5) [49].

|
(5) |

|
Download:
|
| Fig. 2. The linear fits of Langmuir model (a) and Freundlich model (b). | |
If the value of RL is within the range of 0–1, the process is favorable [50]. In these works, all of the RL values were between 0 and 1 which indicated that g-C3N4 was a favorable adsorbent for the removal of ARGs (Table S4 in Supporting information).
We also investigated the influence of different experimental temperatures on the adsorption process. We placed the experimental facility in the working conditions where the temperature has already been set. As shown in Fig. 3a, the reductions of two genes (ampC and ermB) are 3.4–3.6 log and 4.1–4.4 log, respectively. There were no significant differences in the values of each gene among the three temperatures in Figs. 3a and b (P > 0.05). It is obvious that experimental temperature has no effect on the removal of ARGs. This conclusion is in accordance with the findings of Wang and his colleagues [13]. These results also indicate that g-C3N4 powders maintain stable adsorption capability in a wide temperature range of 4-40 ℃.

|
Download:
|
| Fig. 3. Effect of different temperatures on the adsorption capacity (a and b). | |
The pH of the solution is also an important factor in the absorption process. The adsorption efficiency of ARGs on the gC3N4 at the wide pH values ranging from 2.0 to 11.0 is shown in Fig. 4a. Duplicate samples were done for each pH data point. The removals of two ARGs were both significantly affected by the pH of the solution. In this study, acidic or neutral conditions (pH from 2.0 to 7.0) were more beneficial than alkaline condition (pH at 8.0–11.0) for the absorption process. As the pH values increases, the lgC0/C value drops gradually. Obvious reductions of gene copy number with above 4.0 log for ermB and 2.9 log for ampC are observed (2.1 < pH < 5.6). They can also reach over 3.0 log for ermB and 2.0 log for ampC when the pH values are in the range of 5.6-8.8. However, the absorption amounts for both ARGs were obviously reduced at pH > 8.8. It could be seen that the pH of the solution has an obvious effect on the adsorption process. The binding strength between DNA and adsorbent can be conveniently controlled by tuning the solution pH [41]. Li et al. [51] has revealed that DNA strongly bound to multilayer amine modified surfaces under pH 5.6 buffer, when the majority of surface amine groups were protonated. Our investigation results are in agreement with these researches, showing that absorption capacities can be influenced by pH.
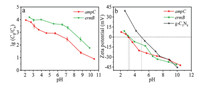
|
Download:
|
| Fig. 4. . Effect of various pH values on the adsorption capacity of g-C3N4 powders (a), and the zeta potentials of g-C3N4 and two ARGs in aqueous suspensions (b). | |
According to previous studies, g-C3N4 and eARGs are both ampholytes with pivotal physicochemical properties. IEP is an essential parameter which is able to characterize the adsorption properties of materials. The surface charge of ampholytes is positive when the pH value of the environment is below its IEP. In contrast, it is negative above its IEP [52]. The zeta potentials were measured to further investigate the surface charge of the g-C3N4 powders in aqueous suspensions and understand the adsorption mechanisms. Triplicate samples were done for each pH effect data point and the average values were presented in Fig. 4b. In the suspension with the initial pH, the zeta potential of g-C3N4 is 39.2 mV. As the pH of aqueous suspensions increases, zeta potentials decrease continuously. The IEP of g-C3N4 is approximate 5.0. At pH < 5.0, the surface charge of the g-C3N4 is positive owing to the protonation reaction, whereas deprotonation reaction occurs and the surface of g-C3N4 turns negative at pH > 5.0. The protonation-deprotonation transition of g-C3N4 in aqueous suspensions involves interactions among hydrogen ions, hydroxyl ions, and certain groups on the g-C3N4 surface. The zeta potential curves obtained for the two ARGs exhibited similar tendency. The zeta potentials of ampC steadily decrease from 7.45 mV (in strong acidic conditions) to -42.4 mV (in strong basic conditions). On the basis of above analysis, the IEP of ampC is about 3.0 and the IEP of ermB is 3.2 or so. When the pH value is above 5.0, the adsorbates and adsorbent are both negatively charged with similar potentials. Electrostatic interaction makes strengthen action in the adsorption process. ARGs should be repelled by the g-C3N4 due to intense electrostatic repulsion. Therefore, the adsorption effect is not ideal.
We have verified that the g-C3N4 powders can be served as column packing. Removal of pollutants in water by an adsorption column is an attractive technique, because it allows non-stop operation [53]. We fabricated a short column to purify water by filtration. A syringe (the volume capacity is 12 mL and the diameter is 1.8 cm) was chosen as the column, and g-C3N4 powders were added into the column. A slice was placed at the bottom to prevent the powders from leaking from the syringe. During the filtration process, the concentration of the effluent and corresponding time were recorded. As shown in Fig. 5a, at the beginning, the concentration of outflow is relatively close to the initial concentration, indicating the adsorption efficiency of the column is unremarkable. The reason for this phenomenon is that the adsorbents (compact g-C3N4 powders) have loose contact with the target ARGs in solutions. The adsorption rate could be limited by the diffusion of adsorbate molecules through the column. The values of lgC0/C gradually increase with the permeation volume. 50 mL solution was fully filtered through the column. The final reductions of ARGs were 1.7 log for ampC and 3.0 log for ermB. As time went on, the adsorption amounts of g-C3N4 powders steadily rose (Fig. 5b). The filtration process was completed in 22 min and the average adsorption rate was 2.3 mL/min. In fact, the total adsorption amounts of g-C3N4 powders by a column were lowerthan those by directly adding g-C3N4 powders to solution. It mainly depended on diffusion and contact between the adsorbent and the adsorbate. The more fully g-C3N4 powders contact with ARGs, the better results the process achieve. The contact time was determined by the flow rate, which could affect the adsorption capacity to a great degree and the effect of flow rate remains further study.
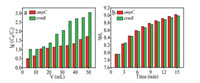
|
Download:
|
| Fig. 5. The differences between the concentration of inflow and outflow as a function of permeation volume (a), and adsorption amounts of the column versus time (b). | |
In conclusion, an adsorption process using g-C3N4 powders as a novel adsorbent was employed to removal eARGs from water. The g-C3N4 powders were successfully prepared in a facile method and convenient for large-scale production. The concentration of two representative ARGs were measured with qPCR technique. ARGs (ampC and ermB) were effectively removed in the adsorption process with a 3.2 log and 4.2 log reductions, compared with other treatment technologies and other adsorption materials, it has good adsorption performance (Text S7 in Supporting information). Langmuir isotherm and pseudo-second-order model were proven to be well fitted with the experimental data. The pH value of the solution could affect the adsorption capacity of g-C3N4 powders. In terms of the absorption process, acidic or neutral conditions (pH from 2.0 to 7.0) were more beneficial than alkaline condition. Furthermore, the adsorbent maintains good adsorption capability in a wide temperature range of 4-40 ℃ and can be served as column packing to purify water by filtration. Based on bulk availability and superior removal performance, g-C3N4 is a promising candidate for the effective adsorption of ARGs from water.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThe authors gratefully acknowledge the financially support by the National Natural Science Foundation of China as general projects (Nos. 21677080 and 21722702), and the Natural Science Foundation of Hebei Province (No. B2019202078).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.08.015.
| [1] |
S.P. Rong, Y.B. Sun, Z.H. Zhao, Chin. Chem. Lett. 25 (2014) 187-192. DOI:10.1016/j.cclet.2013.11.003 |
| [2] |
J. Davies, D. Davies, Microbiol. Mol. Biol. Rev. 74 (2010) 417-433. DOI:10.1128/MMBR.00016-10 |
| [3] |
D.I. Andersson, D. Hughes, FEMS Microbiol. Rev. 35 (2011) 901-911. DOI:10.1111/j.1574-6976.2011.00289.x |
| [4] |
Y. Agersø, D. Sandvang, Appl. Environ. Microb. 71 (2005) 7941-7947. DOI:10.1128/AEM.71.12.7941-7947.2005 |
| [5] |
C. Rodriguez, L. Lang, A. Wang, K. Altendorf, et al., Appl. Environ. Microb. 72 (2006) 5870-5876. DOI:10.1128/AEM.00963-06 |
| [6] |
F.F. Reinthaler, J. Posch, G. Feierl, et al., Water Res. 37 (2003) 1685-1690. DOI:10.1016/S0043-1354(02)00569-9 |
| [7] |
J.C. Chee-Sanford, R.I. Aminov, I.J. Krapac, et al., Appl. Environ. Microb. 67 (2001) 1494-1502. DOI:10.1128/AEM.67.4.1494-1502.2001 |
| [8] |
C.W. Xi, Y.L. Zhang, C.F. Marrs, et al., Appl. Environ. Microb. 75 (2009) 5714-5718. DOI:10.1128/AEM.00382-09 |
| [9] |
J. Li, B. Shao, J.Z. Shen, et al., Environ. Sci. Technol. 47 (2013) 2892-2897. DOI:10.1021/es304616c |
| [10] |
A. Pruden, R. Pei, H. Storteboom, K.H. Carlson, Environ. Sci. Technol. 40 (2006) 7445-7450. DOI:10.1021/es060413l |
| [11] |
C.W. McKinney, A. Pruden, Environ. Sci. Technol. 46 (2012) 13393-13400. DOI:10.1021/es303652q |
| [12] |
K.M. Nielsen, P.J. Johnsen, D. Bensasson, D. Daffonchio, Environ. Biosaf. Res. 6 (2007) 37-53. DOI:10.1051/ebr:2007031 |
| [13] |
D.N. Wang, L. Liu, Z.G. Qiu, et al., Water Res. 92 (2016) 188-198. DOI:10.1016/j.watres.2016.01.035 |
| [14] |
D.Q. Mao, Y. Luo, J. Mathieu, et al., Environ. Sci. Technol. 48 (2014) 71-78. DOI:10.1021/es404280v |
| [15] |
G. Pietramellara, J. Ascher, F. Borgogni, et al., Biol. Fertil. Soils 45 (2009) 219-235. DOI:10.1007/s00374-008-0345-8 |
| [16] |
D.J. Levy-Booth, R.G. Campbell, R.H. Gulden, et al., Soil Biol. Biochem. 39 (2007) 2977-2991. DOI:10.1016/j.soilbio.2007.06.020 |
| [17] |
N.X. Lu, J.L. Zilles, T.H. Nguyen, Appl. Environ. Microb. 76 (2010) 4179-4184. DOI:10.1128/AEM.00193-10 |
| [18] |
B. Zhu, Water Res. 40 (2006) 3231-3238. DOI:10.1016/j.watres.2006.06.040 |
| [19] |
Q.B. Yuan, M.T. Guo, J. Yang, PLoS One 10 (2015) e0119403. DOI:10.1371/journal.pone.0119403 |
| [20] |
M.T. Guo, Q.B. Yuan, J. Yang, Environ. Sci. Technol. 49 (2015) 5771-5778. DOI:10.1021/acs.est.5b00644 |
| [21] |
W.W. He, N. Li, X. Wang, T.L. Hu, X.H. Bu, Chin. Chem. Lett. 29 (2018) 857-860. DOI:10.1016/j.cclet.2017.10.003 |
| [22] |
G.C. Sun, F.Z. Zhang, Q.S. Xie, W. Luo, J.P. Yang, Chin. Chem. Lett. 31 (2020) 1603-1607. DOI:10.1016/j.cclet.2019.10.018 |
| [23] |
Y.H. Wu, Q. Chen, S. Liu, et al., Chin. Chem. Lett. 30 (2019) 2186-2190. DOI:10.1016/j.cclet.2019.08.014 |
| [24] |
Y.Y. Zhang, Z.X. Zhou, Y.F. Shen, ACS Nano 10 (2016) 9036-9043. DOI:10.1021/acsnano.6b05488 |
| [25] |
R. Hu, X.K. Wang, S.Y. Dai, et al., Chem. Eng. J. 260 (2015) 469-477. DOI:10.1016/j.cej.2014.09.013 |
| [26] |
M. Groenewolt, M. Antonietti, Adv. Mater. 17 (2005) 1789-1792. DOI:10.1002/adma.200401756 |
| [27] |
H.S. Zhai, L. Cao, X.H. Xia, Chin. Chem. Lett. 24 (2013) 103-106. DOI:10.1016/j.cclet.2013.01.030 |
| [28] |
S. Hwang, S. Lee, J.S. Yu, Appl. Surf. Sci. 253 (2007) 5656-5659. DOI:10.1016/j.apsusc.2006.12.032 |
| [29] |
D.Y. Ni, Y.Y. Zhang, Y.F. Shen, S.Q. Liu, Y.J. Zhang, Chin. Chem. Lett. 31 (2020) 115-118. DOI:10.1016/j.cclet.2019.04.068 |
| [30] |
M.X. Ran, P. Chen, J.R. Li, et al., Chin. Chem. Lett. 30 (2019) 875-880. DOI:10.1016/j.cclet.2019.03.016 |
| [31] |
F. Dong, L.W. Wu, Y.J. Sun, et al., J. Mater. Chem. 21 (2011) 15171-15174. DOI:10.1039/c1jm12844b |
| [32] |
M. Ding, J.J. Zhou, H.C. Yang, et al., Chin. Chem. Lett. 31 (2020) 71-76. DOI:10.1016/j.cclet.2019.05.029 |
| [33] |
Q. Liu, D.B. Zhu, M.L. Guo, Y. Yu, Y.J. Cao, Chin. Chem. Lett. 30 (2019) 1639-1642. DOI:10.1016/j.cclet.2019.05.058 |
| [34] |
Y.P. Chen, H.Y. Zhang, R. Lu, A.C. Yu, Chin. Chem. Lett. 29 (2018) 543-546. DOI:10.1016/j.cclet.2017.09.022 |
| [35] |
F. Dong, Z.Y. Wang, Y.H. Li, et al., Environ. Sci. Technol. 48 (2014) 10345-10353. DOI:10.1021/es502290f |
| [36] |
Q.K. Chen, L. Chen, J.J. Qi, et al., Chin. Chem. Lett. 30 (2019) 1214-1218. DOI:10.1016/j.cclet.2019.03.002 |
| [37] |
T. Schwartz, W. Kohnen, B. Jansen, U. Obst, FFEMS Microbiol. Ecol. 43 (2003) 325-335. DOI:10.1111/j.1574-6941.2003.tb01073.x |
| [38] |
A. Di Cesare, D. Fontaneto, J. Doppelbauer, G. Corno, Environ. Sci. Technol. 50 (2016) 10153-10161. DOI:10.1021/acs.est.6b02268 |
| [39] |
Y. Li, M.Q. Li, J. Zhang, X.Y. Xu, Chin. Chem. Lett. 30 (2019) 762-766. DOI:10.1016/j.cclet.2018.11.005 |
| [40] |
L. Fan, C. Luo, X. Li, et al., J. Hazard. Mater. 215- 216 (2012) 272-279. |
| [41] |
M. Wu, R. Kempaiah, P.J. Huang, et al., Langmuir 27 (2011) 2731-2738. DOI:10.1021/la1037926 |
| [42] |
H.X. Li, L.J. Rothberg, J. Am. Chem. Soc. 126 (2004) 10958-10961. DOI:10.1021/ja048749n |
| [43] |
H.X. Li, L.J. Rothberg, Anal. Chem. 76 (2004) 5414-5417. DOI:10.1021/ac049173n |
| [44] |
S.J. Sowerby, C.A. Cohn, W.M. Heckl, N.G. Holm, Proc. Natl. Acad. Sci. U. S. A. 98 (2001) 820-822. DOI:10.1073/pnas.98.3.820 |
| [45] |
S. Gowtham, R.H. Scheicher, R. Ahuja, R. Pandey, S. Karna, , Phys. Rev. B 76 (2007) 033401. |
| [46] |
X.Y. Sun, P.Z. Zhang, B. Ai, Y.B. Wang, Chin. Chem. Lett. 27 (2016) 139-144. DOI:10.1016/j.cclet.2015.08.008 |
| [47] |
M.J. Chang, W.N. Cui, J. Liu, K. Wang, X.J. Chai, J. Mater. Sci. Mater. Electron. 29 (2018) 6771-6778. DOI:10.1007/s10854-018-8663-6 |
| [48] |
Y.S. Ho, G. McKay, Water Res. 34 (2000) 735-742. DOI:10.1016/S0043-1354(99)00232-8 |
| [49] |
M.S. Chiou, P.Y. Ho, H.Y. Li, Dyes Pigm. 60 (2004) 69-84. DOI:10.1016/S0143-7208(03)00140-2 |
| [50] |
M. Wu, R. Kempaiah, P.J. Huang, V. Maheshwari, J. Liu, Langmuir 27 (2011) 2731-2738. DOI:10.1021/la1037926 |
| [51] |
Z. Li, K.M. Ashraf, M.M. Collinson, D.A. Higgins, Langmuir 33 (2017) 8651-8662. DOI:10.1021/acs.langmuir.7b00044 |
| [52] |
M. Wang, Y.X. Liu, D. Li, J.W. Tang, W.X. Huang, Chin. Chem. Lett. 30 (2019) 985-988. DOI:10.1016/j.cclet.2019.01.017 |
| [53] |
Y.Q. Chen, L.B. Chen, H. Bai, L. Lei, J. Mater. Chem. A 1 (2013) 1992-2001. DOI:10.1039/C2TA00406B |
 2020, Vol. 31
2020, Vol. 31 

