b School of Civil Engineering, Guangzhou University, Guangzhou 510006, China;
c School of Civil and Architectural Engineering, Guizhou University of Engineering Science, Bijie 551700, China;
d School of Environmental Science and Engineering, Guangzhou University, Guangzhou 510006, China;
e State Key Laboratory of Organic Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, China;
f Environmental Engineering Program, Department of Civil Engineering, Auburn University, Auburn, AL 36849, United States;
g School of Environmental Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
With rapid economic growth, industrialization and urbanization worldwide, a multitude of pollutants are introduced into the aquatic environment, such as heavy metals (HMs) [1], polycyclic aromatic hydrocarbons (PAHs) [2], halogenated flame retardants (HFRs) [3], and pharmaceutically active compounds (PhACs) [4], posing significant threats to the environmental and human health. As more and more sewage treatment plants (STPs) are built, the discharge of sewage effluents has become one of the principal routes introducing residual pollutants into the aquatic environment [5]. Therefore, STPs can greatly impact the surrounding ecological environment.
Biological treatment is the dominant process to treat sewage, which includes activated sludge process (ASP) and biofilm process. Currently, activated sludge technology is the most widely used process for secondary wastewater treatment [6]. ASP utilizes microorganisms, particularly bacteria, for the degradation of biodegradable substrates. Depending on the design and the specific, an activated sludge STP can effectively break down organic carbonaceous substrates and nutrients [7]. The A2/O process, consisting of anaerobic, anoxic and oxic units in succession, is one of the most widely used ASPs because of its capability for simultaneous removal of biochemical oxygen demand (BOD) and nutrients [8]. To reduce energy consumption and prevent the potential interference of nitrate removal with phosphorus release, a so-called reversed A2/O process was developed by canceling the internal recycle and reversing the order of the anaerobic and anoxic units [8]. Since then, the reversed A2/O process has been applied in many STPs around the world.
Yet, studies on A2/O process have been focused on removal of bulk chemical oxygen demand (COD), BOD, and nutrients [9], and the removal of various types of pollutant species including the micro- and emerging pollutants by A2/O process has been seldom addressed. As more and more emerging pollutants enter the environment through STP effluents, and with the wide application of the conventional or revised A2/O process, it is direcly needed to evaluate the performance of the technology for removal of various types of important pollutants.
In this study, we investigated the occurrence, removal and fate of targeted pollutants species across a reversed A2/O process in Guangzhou Datansha Sewage Treatment Plant. The targeted pollutants included 3 types, i.e., polybrominated diphenyl ethers (PBDEs) and some novel HFRs, sulfonamide antibiotics (SAs), and HMs. PBDEs are man-made aromatic chemicals and have been used as additive fire retardants in a wide range of products, such as polyurethane foam, electronic products, nylon, textile [10]. The high hydrophobicity and halogenation of PBDEs have led to their wide extent of occurrence in STPs [11]. Due to the environmental ubiquity and potential adverse health effects, some PBDEs (i.e., commercial PentaBDE and OctaBDE formulations) were banned by the European Union in 2004, and California and Hawaii in 2006 [10]. However, many so-called novel HFRs were introduced to replace PBDEs, such as decabromodiphenyl ethane (DBDPE), 1, 2-bis(2, 4, 6-tribromophenoxy)ethane (BTBPE), and pentabromotoluene (PBT). Recent studies have suggested that the concentrations of novel HFRs in environmental media have shown an increasing trend [3]. For example, Wu et al. indicated the concentration of DBDPE in the sewage sludge ranged from 680 ng/g to 27, 400 ng/g [11]. The SAs have been a wide spectrum of antimicrobial action and widely used in animal husbandry. Due to high excretion rates, easy transfer, and lack of appropriate treatment, residues of SAs have been detected in a variety of environmental media [12]. A recent study reported the maximum concentrations of sulfadiazine and sulfamethoxazole in the STP influents reached more than 200 ng/L [13]. HMs are well known environmental pollutants that affect both human and ecological health [1]. Yang et al. collected sludge samples from 107 STPs in China, and reported the average concentrations of As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn were 20.2, 1.97, 93.1, 218.8, 2.13, 48.7, 72.3, and 1058 mg/kg, respectively [14].
The overall goal of this work was to investigate the performance of reversed A2/O process for removal of multiple pollutants in sewage. The specific objectives were to: (1) identify the critical treatment units for pollutants removal, (2) compare the treatment performance of the conventional and reversed A2/O processes, (3) characterize the removal patterns of different kinds of pollutants, and (4) investigate the partition of pollutants between aqueous and solid phases.
Datansha Sewage Treatment Plant (DSTP) is the first large-scale STP in Guangzhou, located in Datansha Island in the western suburb of Guangzhou (23°07'24.5"N, 113°13'10.9"E, Fig. 1a). It covers an area of 25 hectares, and has a total designed treatment capacity of 550, 000 tons per day (TPD). It mainly treats sewages from Liwan District, Yuexiu District, and Baiyun District with a service area of 89.7 square kilometers, and a population of approximately 1, 427, 000.

|
Download:
|
| Fig. 1. (a) Location of Datansha Sewage Treatment Plant (DSTP), and (b) schematic of the reversed A2/O process and sampling locations. | |
DSTP has three parallel treatment lines. The first two lines employ the A2/O process with a processing capacity of 150, 000 TPD each.The third line employs the reversed A2/O process with a processing capacity of 250, 000 TPD. The STP was operated to comply with the Chinese "Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant" (GB 18918-2002). Fig. 1b shows the schematic of the reversed A2/O process. The basic feed water quality parameters are: BOD5 = 120 mg/L, CODcr = 250 mg/L, SS = 150 mg/L, NH4- N = 30 mg/L, and TP = 4 mg/L. The raw water is first passed through the fine grids followed by a sedimentation basin to remove the large amount of suspended solids. The primary effluent is then introduced into the reversed A2/O bioreactors, and subsequently separated with the sludgein the secondary settling tank.Afterpost-chlorination, the final effluent is discharged into the Pearl River with the typical water quality being: BOD5 = 20 mg/L, CODcr = 60 mg/L, SS = 20 mg/L, NH4- N = 10 mg/L, and TP = 0.5 mg/L.
In this study, duplicate water samples were collected at 5 locations of the reversed A2/O process (Fig. 1b), i.e., influent water (L1), effluent from the sedimentation basin (L2), water in the middle oxic basin (L3), effluent from the secondary setting tank (L4), and final effluent (L5). For analyses of PBDEs, novel HFRs and SAs, 10 L of each water sample was individually collected in precleaned Ampere glass bottles. For HMs analyses, approximately 100 mL of water sample was collected in polypropylene bottles and then filtered with a 0.45-mm microporous membrane; the pH was adjusted to < 2 with concentrated nitric acid. For comparison, water samples were also collected at the same sampling locations of the conventional A2/O process. All the samples were put into icepacked coolers and transported back to the laboratory for immediate processing.
Eighteen PBDE congeners (i.e., BDE 28, 47, 66, 85, 99, 100, 138, 153, 154, 183, 196, 197, 202, 203, 206, 207-209, Table S1 in Supporting information) and 11 novel HFRs (i.e., anti- and syndechlorane plus (DP), decachloropentacyclooctadecadiene (aCl10DP), undecachloropentacyclooctadecadiene (aCl11DP), tetrabromo-p-xylene (pTBX), PBT, pentabromoethylbenzene (PBEB), 2, 2', 4, 4', 5, 5'-hexabromobiphenyl (PBB 153), hexabromobenzene (HBB), BTBPE and DBDPE, Table S2 in Supporting information) were analyzed for water and particulate phases referencing the reported methods with modifications [15]. Detailed analytical procedure and quality assurance/control (QA/QC) are given in Text S1 (Supporting information).
Eight SAs, i.e., sulfadiazine (SDZ), sulfathiazole (STZ), sulfapyridine (SPD), sulfamethyldiazine (SMD), sulfamethoxydiazine (SMOD), sulfadimidine (SDD), sulfamethizole (SMZ), and sulfamethoxazole (SMX) (Table S3 in Supporting information) in the water samples were analyzed using liquid chromatography coupled with electrospray tandem mass spectrometry (LC–MS/ MS) (Agilent 6490, U. S. A.). The details for analysis of SAs are described in Text S2 (Supporting information). The calibration curve was prepared within a range of concentrations (0.1–50 μg/L), where a strong linear relationship was obtained (R2 > 0.99) (Table S4 in Supporting information).
For HMs, Mn, Cr, Pb, Ni, Cu and Zn were determined by inductively coupled plasma atomic emission spectroscopy (ICPOES) (PerkinElmer, Wellesley, Massachusetts, USA), and Cd was measured with an AAnalyst 800 atomic absorption spectrometer (Perkin-Elmer, U. S. A.).
In the influent wastewater, 5 PBDEs (BDE 153, 206-209) were detected with a total concentration of 32.26 ± 2.62 ng/L and 22.94 ±10.53 ng/L in the water phase and particulate phase, respectively (Table S5 in Supporting information). The total PBDEs concentrations (sum in both phases, 55.20 ± 11.19 ng/L) were comparable with the recently reported values (30.4-66.1 ng/L) from STPs in Hong Kong, China [16], but much lower than the previous reported values for DSTP (568 ng/L) [17] and other reported values (265 ± 210 ng/L) in Canadian STPs [18, 19], which is consistent with the fat that the usage of PBDE flame retardants was reduced in the past several years in the Pearl River Delta (PRD). In terms of PBDE congeners, BDE 209 (predominant composition of DecaBDEs) accounted for 84.41% of the total PBDEs, which was similar to Peng's report [17]. This indicates DecaBDEs are still the dominant PBDE flame retardants. However, the previously frequently detected BDE 47 and 49 (dominant PentaBDE compositions) in raw sewages were not detected in the present study, indicating PentaBDEs were rarely used. These results agree with the PBDEs production in China: OctaBDEs were never manufactured in China, and Chinese producers stopped production of commercial PentaBDEs in 2004; although the use of DecaBDEs is not subject to any regulatory action in China, the domestic production of DecaBDEs decreased from approximately 41, 500 tons in 2005 to 20, 500 tons in 2011 [20].
Three novel HFRs (anti-DP, syn-DP and DBDPE) were detected in the particulate phase of the raw sewage with an average concentration of 19.02 ±11.45 ng/L (Table S6 in Supporting information). The ratio of novel HFRs to PBDEs reached 0.34 ± 0.16, indicating these alternative flame retardants were largely used in local area. Compared with PBDEs, anti-DP, syn-DP and DBDPE have higher octanol-water partition coefficient (KOW) (Tables S1 and S2). Therefore, these novel HFRs prefer to partition in the particulate phase. DP is a highly chlorinated flame retardant with a similar structure to the pesticide mirex (Table S2), and was suggested as a substitute to DecaBDEs in polymeric applications since the 1960s [21]. The observed DP concentration including both isomers (7.5 ng/L) in the influent of DSTP was an order of magnitude higher than the reported value (0.46 ng/L) in a STP in Shanghai, China [22], which can be due to the wide distribution of manufacturing industries of electronic products and dumping sites of e-wastes in the PRD region [17]. For the two DP isomers, the antiDP fractional abundance (fanti) is generally used to elucidate the behavior and fate of DP in the environment, which is expressed as fanti = [anti-DP]/([anti-DP] + [syn-DP]) [21]. In this study, the average fanti value of the raw sewage was 0.37, which is significantly lower than that for the commercial DP in the Chinese market (fanti = 0.59), indicating a stereoselective depletion of DP isomers during their transport to the STPs. DBDPE is structurally similar to BDE209 (Tables S1 and S2), and has been introduced into the market as a principal alternative for DecaBDEs since the early 1990s [21]. Compared with the reported concentrations (0– 23.6 ng/L) in influents of other STPs worldwide [23-25], the DBDPE concentration (11.52 ±11.65 ng/L) in DSTP's influent was at a relatively higher level.
The overall removal of PBDEs (60.5% ± 4.3%) through the reversed A2/O process was much lower than that of the novel HFRs (98.4% ± 2.8%) (Tables S5 and S6), which can be attributed to the higher concentration and lower Kow of PBDEs relative to DP and DBDPE. While both adsorption to suspended particulate matter (SPM) and biodegradation can remove HFRs in the treatment process, adsorption is considered the chief mechanism duo to the refractory nature of HFRs to microbial metabolism [3]. With respect to individual PBDE congeners, BDE 209 in the particulate phase of final effluent showed a high residual concentration (13.41 ±5.18 ng/L), resulting a low removal efficiency (32.1% ± 9.8%). Therefore, the removal of BDE 209 in the particulatematter from the reversed A2/O process should be further studied. For the two DP isomers, syn-DP was completely removed, while anti-DP showed a removal efficiency of 79.0% ± 36.3%, indicating the reversed A2/O process is selective toward the syn-conformation of DP.
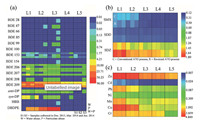
Fig. 2a shows the concentrations of PBDEs and novel HFRs across the reversed A2/O process at locations L1-L5. The total HFRs (PBDEs plus novel HFRs) concentrations in the water phase decreased along the treatment process. However, the total HFRs concentrations in the particulate phase significantly increased across the primary treatment and activated sludge bioreactors (ASBs) (L2 and L3) due to the increased content of SPM. After the secondary setting tank (L4), the HFRs sharply decreased with the removal of SPM. The secondary setting tank removed the most HFRs in both water and particulate phases (by 37.3% and 88.6%, respectively), indicating the sedimentation is a critical unit for controlling the HFRs level in the effluent.
|
Download:
|
| Fig. 2. Concentration distribution of multiple pollutants across the reversed A2/O process in DSTP, Guangzhou, China. (a) PBDEs and novel HFRs (ng/L), (b) SAs (μg/L), and (c) HMs (mg/L). | |
Besides the highly brominated PBDEs (BDE 206-209) detected in the raw sewage, several less brominated PBDE congeners were detected in the particulate phase of water samples collected from oxic basin (L3) including BDE 28, 47, 66, 85, 99, 100 and 154 (Fig. 2a). This observation indicates that nona- and deca-BDEs were degraded to tri-, tetra-, penta- and hexa-BDEs by microbes in the particulate phase. However, the produced less brominated PBDEs accounted for only 0.6% of the total PBDEs, suggesting a limited role of biodegradation in removal of PBDEs. A noticeable enrichment of DBDPE in the particulate matter of ASBs (80.58 ± 19.98 ng/L) was observed (Fig. 2a), indicating the high affinity of DBDPE toward the solids and resistance to biodegradation. Therefore, caution shouldbe exercised in the application or handling of the DBDPE-laden biosolids to avoid the toxic effects to the environmental and human health. Unlike other HFRs, DP evidently decreased in the particulate phase of ASBs (Fig. 2a), indicating DP was readily degraded or transformed to other intermediates via microbial activity.
SAs are a big family of sulfonamide-related antibiotics commonly used in clinical and veterinary medicine and have drawn increasing attention worldwide. In this study, SDZ, SDD and SMX were detected in the influents of DSTP (Table S7 in Supporting information). SMX is one of the most frequently reported SAs and the detected concentration in the influents of DSTP (0.37–0.48 μg/L) was generally lower than reported values from other STPs in U. S. A. (1.09–1.47 μg/L) [26, 27] or Canada (0.65 μg/L) [28]. However, the concentrations of SDZ (1.40–1.80 μg/L) and SDD (0.27–0.36 μg/L) in the influents of DSTP were much higher than those from other STPs in U. S. A. (< 0.1 μg/L) [26, 27], indicating SDZ and SDD were largely used in the local area.
Biodegradation and adsorption are considered the two main removal mechanisms for organic pollutants during secondary treatments in STPs [29]. For SAs, the distribution coefficient (Kd) values found in the literature are typically low (< 500 L/kg), and adsorption contributes to < 10% of the overall removal [30]. The overall removal was specific for SDZ, SDD and SMX in the DSTP (Table S7). SDZ and SDD showed modest removal (8%-29%), while the removal of SMX was much higher (51%-74%). The RE of total SAs in conventional or reversed A2/O process was comparable (22%– 33%). Therefore, the order of the anaerobic and anoxic units may not affect the biodegradation of hydrophilic organic pollutants like SAs. Compared with other reports (Table S7), the removal of SAs varied a lot for different STPs. Thus, the specific microbial conditions may pose a great effect on the removal of SAs. In addition, García Galán and coworkers (2012) indicated that membrane bioreactor (MBR) treatment was more efficient for removing SAs than ASP [29]. However, their results are confined with the special membrane materials and microorganism populations, and more work is needed to elucidate the underlying mechanisms.
Based on the observed removal of SAs in different treatment units (Fig. 2b), SDZ and SDD were recalcitrant to biodegradation, and nearly no removal (< 1.0%) was observed in the ASBs (L3); however, SMX showed a higher biodegradability and the highest removal percentage (40.4%) was found when in the bioreactors. Both SDZ and SDD have the pyrimidin moiety while SMX has the structure of isoxazol (Table S3). The difference in molecular structure may result in the different biodegradabilities of SAs. Many studies have also reported the selectivity of STPs on removal of SAs [26, 29].
HM pollution has become serious in China, and the major rivers and lakes are reportedly polluted by heavy metals at different levels, with up to 80.1% of sediments being polluted [1]. In the raw sewage of DSTP, high concentrations of Cd (0.020 ± 0.002 mg/L), Cr (1.52 ± 0.01 mg/L), Cu (3.52 ± 0.20 mg/L), Mn (1.61 ±0.09 mg/L), Ni (0.35 ± 0.02 mg/L), Pb (1.56 ± 0.09 mg/L) and Zn (4.64 ± 0.28 mg/L) were found (Table S8 in Supporting information). For comparison, Table S8 also gives the reported values elsewhere [31-34].
The reversed A2/O process of DSTP showed high removal efficiency for HMs and the overall removal of total HMs achieved 70.1% ± 1.2% (Table S8). The relatively high removal efficiency of HMs may be related to their relatively high input concentrations. ASP is known effective for removing HMs from wastewater, and the primary mechanisms include sorption by inorganic particles, biosorption by biosolids (biopolymers or biomass), and precipitation as metal hydroxides [31, 32]. The removal of HMs by STPs appeared selective (Table S8). Mn and Ni exhibited the lowest removal efficiencies (REs) (47.5% ± 2.2% and 35.0% ± 2.6%, respectively) while Cr and Cu were most effectively removed in DSTP with the REs being > 80%. This can be attributed to the selective adsorption of these metals onto inorganic particles and biomass during the treatment process [34].
In terms of different treatment units of the reversed A2/O process, ASBs were the most effective units for removal of Cu (70.9%), Pb (59.1%), Cd (43.7%) and Zn (42.2%) (Fig. 2c). The sorption/biosportion on inorganic particles and biomass played a critical role during the process. Approximately 80% of Cr was removed by chlorination (L5). Chlorination increased the water pH, resulting in precipitation of Cr hydroxides [35].
Although the multiple pollutants have very different structures and properties, the dominant removal mechanisms of various pollutants in reversed A2/O process are similar, i.e., physiochemical sorption, and biological sorption, and biodegradation. Therefore, similar removal pattern may be expected among multiple pollutants. Bicluster analysis based on the REs across the reversed A2/O process indicated several clusters among these pollutants (Fig. 3a). SDZ and SDD, BDE 206 and 207, Cu and Cd showed the most similar removal trends, which were also confirmed by the significant positive Pearson correlation coefficients (Table S9 in Supporting information). SDZ and SDD have the same pyrimidin structure and both were recalcitrant to biodegradation. BDE 206 and 207 are congeners with similar structure and properties. Moreover, their concentration levels were comparable in the raw sewage (2.26–3.62 ng/L), resulting in their similar removal pattern. Cu and Cd showed similar adsorption capacities and had relatively high potential to be adsorbed on particles/solids. In addition, Ni and BDE 209, Zn and SMX, Mn and BDE 153 also showed similar removal patterns. Ni and BDE 209 had relatively low REs and most removal occurred in the secondary settling tank. Zn and SMX displayed higher biological removal (biodegradation and biosorption). Both Mn and BDE 153 showed negative removal after secondary settling tank. Mn was easily re-released during secondary settling. However, the accumulation of BDE 153 in L4 samples may come from the degradation products from higher bromated PBDEs. Similar results were observed for principal components analysis (PCA), revealing similar pollutant associations (Fig. 3b and Table S10 in Supporting information).

|
Download:
|
| Fig. 3. Bicluster analysis (a) and principal components analysis (b) of removal efficiencies of multiple pollutants across the reversed A2/O process in DSTP, Guangzhou, China. | |
In terms of treatment units in the reversed A2/O process, ASBs showed the highest average RE (27.8%) for the multiple pollutants, where SMX, Zn, Cu, Cd and Pb were most effectively removed (Fig. 3a). The primary sedimentation also played an important role in reducing the pollutant loadings (26.2%), where Mn, SDZ and SDD were most effectively removed. The post-chlorination unit contributed to an average of 23.9% to the overall removal of the multiple pollutants, including PBDEs, SMZ, Cr and Zn. The addition of chlorine greatly facilitated removal of Cr and Zn through the precipitation of metal hydroxides at neutral or alkaline pH. Chlorination may also break down the structures of PBDEs and SMZ. However, the application of chlorine can produce certain health risks. Chlorine is capable of converting Cr(Ⅲ) to Cr(Ⅵ), which is highly toxic and has been categorized as a human carcinogen [35]. Chlorination of organic matters in the effluents can result in the formation toxic disinfection byproducts (DBPs), which have drawn great concerns worldwide [36]. The secondary sedimentation is important for the separation of SPM from the water phase, and most of the pollutants adsorbed on the solids are removed as excess sludge. However, the addition of chemical agents may cause the re-release of some metal ions, such as Mn and Pb.
In addition, the sorption onto suspended solids is the dominant removal mechanism for PBDEs and HMs during the primary and secondary treatments (Fig. 2). However, the specific sorption mechanisms are different in different treatment stages due to different properties and nature of suspended solids. In the primary treatments, the suspended solids are mainly inorganic particles, and therefore the sorption is physiochemical sorption based on the interaction with the organic matters on suspended particles. In the secondary treatment stage, the suspended solids are sludge particles, which contain the large amount of live and dead microorganisms as well as the secreted biomacromolecules including fats, proteins, amino acids, sugars, carbohydrates, lignin, celluloses and fatty acids. The sorption of pollutants onto sludge particles can be ascribe to biological sorption based on the interaction with biomass or biofilm on sludge.
In conclusion, multiple pollutants including HFRs, SAs and HMs were detected in the raw sewages of a major STP in Guangzhou, China at relatively high levels. The STPs are important for the protection of local ecosystems in PRD as barriers. The A2/O process and its modified processes have been operated in the PRD area for nearly two decades. The present study provided useful information on the removal capacities of reversed A2/O process for multiple pollutants. Generally, the reversed A2/O process demonstrated good REs for the targeted pollutants and each treatment unit played important roles. The primary sedimentation removed the large particles and reduced the pollutants mass loading. ASBs showed the highest average RE, and many pollutants (e.g., SMX, Zn, Cu, Cd and Pb) achieved the most removal in the bioreactors. The secondary sedimentation is a critical unit as separation of SPM can govern the overall removal effectiveness for pollutants. The postchlorination unit also contributed to the removal of specific pollutants. However, this study also revealed some issues that need to be further investigated: (1) high residual concentration of BDE 209 in the SPM of the treated effluents, (2) high enrichment and biodegradation recalcitrance of DBDPE in particulate matter of ASBs, (3) low REs of specific pollutants such as SDZ, SDD, Mn and Ni, and (4) re-release of Mn and Pb during the secondary sedimentation. The information can be used to improve the operation and design of conventional and modified A2/O processes for removal of multiple pollutants including emerging and micropollutants in municipal wastewater.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis project was supported by the National Natural Science Foundation of China (No. 91851110), Guangzhou University's 2017 Training Program for Young Top-Notch Personnels (No. BJ201713), Scientific Research Project of Guangzhou University (No. YK2020017) and Guizhou Provincial Department of Education Youth Science and Technology Talents Growth Project (No. KY [2017]300).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.06.029.
| [1] |
J. Fu, C. Zhao, Y. Luo, et al., J. Hazard. Mater. 270 (2014) 102-109. DOI:10.1016/j.jhazmat.2014.01.044 |
| [2] |
J. Fu, S. Sheng, T. Wen, et al., Ecotoxicology 20 (2011) 940-950. DOI:10.1007/s10646-011-0622-4 |
| [3] |
Q. Wu, Y. Du, Z. Huang, et al., Sci. Total Environ. 669 (2019) 1001-1010. DOI:10.1016/j.scitotenv.2019.03.178 |
| [4] |
J. Fu, W.N. Lee, C. Coleman, et al., Sci. Total Environ. 664 (2019) 240-248. DOI:10.1016/j.scitotenv.2019.02.026 |
| [5] |
H. Hou, L. Duan, B. Zhou, et al., Chin. Chem. Lett. 31 (2020) 543-546. DOI:10.1016/j.cclet.2019.08.031 |
| [6] |
M. Shahzad, S. Khan, P. Paul, Water 7 (2015) 855-867. DOI:10.3390/w7030855 |
| [7] |
U. Sarkar, D. Dasgupta, T. Bhattacharya, S. Pal, T. Chakroborty, Desalination 252 (2010) 120-126. DOI:10.1016/j.desal.2009.10.014 |
| [8] |
R. Qi, T. Yu, Z. Li, et al., J. Environ. Sci. 24 (2012) 571-578. DOI:10.1016/S1001-0742(11)60808-5 |
| [9] |
S.B. Kwon, D.I. Kim, Y.T. Guan, S. Dockko, Desalin. Water Treat. 54 (2015) 1090-1097. DOI:10.1080/19443994.2014.922447 |
| [10] |
X.T. Wang, L. Chen, X.K. Wang, et al., Chemosphere 133 (2015) 22-30. DOI:10.1016/j.chemosphere.2015.02.064 |
| [11] |
Q. Wu, H. Li, D.T. Kuo, et al., Environ. Pollut. 220 (2017) 63-71. DOI:10.1016/j.envpol.2016.09.023 |
| [12] |
C.C. Yang, C.L. Huang, T.C. Cheng, H.T. Lai, Int. Biodeter. Biodegr. 102 (2015) 116-125. DOI:10.1016/j.ibiod.2015.01.015 |
| [13] |
J. Cui, L. Fu, B. Tang, et al., Sci. Total Environ. 709 (2020) 136192. DOI:10.1016/j.scitotenv.2019.136192 |
| [14] |
J. Yang, M. Lei, T. Chen, et al., Front. Environ. Sci. Eng. 8 (2014) 719-728. DOI:10.1007/s11783-013-0600-6 |
| [15] |
B. Mai, S. Chen, X. Luo, et al., Environ. Sci. Technol. 39 (2005) 3521-3527. DOI:10.1021/es048083x |
| [16] |
Y.B. Man, K.L. Chow, M. Man, et al., Sci. Total Environ. 505 (2015) 261-268. DOI:10.1016/j.scitotenv.2014.09.070 |
| [17] |
X. Peng, C. Tang, Y. Yu, et al., Environ. Int. 35 (2009) 303-309. DOI:10.1016/j.envint.2008.07.021 |
| [18] |
S. Rayne, M.G. Ikonomou, J. Environ. Eng. Sci. 4 (2005) 353-367. DOI:10.1139/s04-071 |
| [19] |
S. Rayne, M.G. Ikonomou, J. Environ. Eng. Sci. 4 (2005) 369-383. DOI:10.1139/s04-067 |
| [20] |
K. Ni, Y. Lu, T. Wang, et al., J. Environ. Manage. 115 (2013) 114-123. DOI:10.1016/j.jenvman.2012.09.031 |
| [21] |
J. Kim, M.H. Son, E.S. Shin, S.D. Choi, Y.S. Chang, Environ. Pollut. 212 (2016) 330-336. DOI:10.1016/j.envpol.2016.01.085 |
| [22] |
N. Xiang, L. Chen, X.Z. Meng, et al., Sci. Total Environ. 487 (2014) 342-349. DOI:10.1016/j.scitotenv.2014.04.014 |
| [23] |
M. Kim, P. Guerra, M. Alaee, S.A. Smyth, Environ. Sci. Pollut. Res. 21 (2014) 13394-13404. DOI:10.1007/s11356-014-3262-4 |
| [24] |
E.D. Schreder, M.J. La Guardia, Environ. Sci. Technol. 48 (2014) 11575-11583. DOI:10.1021/es502227h |
| [25] |
J.R. Nyholm, R. Grabic, H.P.H. Arp, T. Moskeland, P.L. Andersson, Sci. Total Environ. 443 (2013) 307-314. DOI:10.1016/j.scitotenv.2012.10.081 |
| [26] |
P. Gao, M. Munir, I. Xagoraraki, Sci. Total Environ. 421- 422 (2012) 173-183. |
| [27] |
S. Yang, J. Cha, K. Carlson, J. Chromatogr. A 1097 (2005) 40-53. DOI:10.1016/j.chroma.2005.08.027 |
| [28] |
E.L. McClure, C.S. Wong, J. Chromatogr. A 1169 (2007) 53-62. DOI:10.1016/j.chroma.2007.08.062 |
| [29] |
M. García Galán, M.S. Díaz-Cruz, D. Barceló, Anal. Bioanal. Chem. 404 (2012) 1505-1515. DOI:10.1007/s00216-012-6239-5 |
| [30] |
T.A. Ternes, N. Herrmann, M. Bonerz, et al., Water Res. 38 (2004) 4075-4084. DOI:10.1016/j.watres.2004.07.015 |
| [31] |
A. da Silva Oliveira, A. Bocio, T. Beltramini Trevilato, et al., Environ. Sci. Pollut. Res. 14 (2007) 483-489. DOI:10.1065/espr2006.10.355 |
| [32] |
G. Gulyas, V. Pitas, A. Karpati, et al., Environ. Eng. Manag. J. 13 (2014) 2039-2044. DOI:10.30638/eemj.2014.226 |
| [33] |
L.C.F. Souza, F.B. Canteras, S. Moreira, Radiat. Phys. Chem. 95 (2014) 342-345. DOI:10.1016/j.radphyschem.2013.01.025 |
| [34] |
J. Edokpayi, J. Odiyo, T. Msagati, E. Popoola, Int. J. Environ. Res. Pub. Heal. 12 (2015) 7300. DOI:10.3390/ijerph120707300 |
| [35] |
D.R. Lindsay, K.J. Farley, R.F. Carbonaro, J. Environ. Monitor. 14 (2012) 1789-1797. DOI:10.1039/c2em00012a |
| [36] |
J. Fu, W.N. Lee, C. Coleman, et al., Water Res. 123 (2017) 224-235. DOI:10.1016/j.watres.2017.06.073 |
 2020, Vol. 31
2020, Vol. 31 

