b Key Laboratory of Optical Detection Technology for Oil and Gas, China University of Petroleum, Beijing 102249, China
The shortage of fossil energy resources and the global climate warming is threatening the living environment of human beings. The photocatalysis technology for carbon dioxide (CO2) reduction to sustainable hydrocarbon energy may perfectly addresses the above conundrums [1-8]. However, the artificial photosynthesis technique is faced with the low solar energy conversion efficiency as well as the rare products [9-12]. Thus, it is significant to develop high performance photocatalyst. The perovskite-type SrTiO3 possesses the wide band gap whose conduction band is positive enough to reduce CO2, hence it was been widely researched for photoreduction reaction [13-19]. In addition, CO2 as the anhydride of carbonic acid is more likely to adsorb with alkalis strontium on the surface of SrTiO3 [20-22], so that it is promising to further investigate. However, the wide band gap of SrTiO3 plays a doubleedged sword, which can not only decrease the recombination of electron-hole pairs, but also restrains the visible light absorption and the photoexcitation. Thus, the pristine SrTiO3 is faced with the low quantum utilization and less multi-electron products [23, 24]. The strategies should be proposed to optimize SrTiO3 and construct a novel catalyst with outstanding photoreduction performance as well as multi-electrons product (CH4) selectivity.
Three-dimensional ordered macroporous (3DOM) catalysts as photonic crystals have been extensively studied in the field of photocatalysis due to their slow light efficiency [25-31]. Photonic crystals have periodic refractive modulation in the optical wavelength range, which can cause stopband reflection through the Bragg diffraction of the material and prohibit light propagation at certain wavelengths. The contact time between the photon and the material was extended, and the incident light of a specific wavelength is stored in 3DOM materials, thus the light capturing efficiency could be improved. And 3DOM structure could be obtained by replicating a classic face-centered cubic opal structure template [32-34]. In addition, tin disulfide (SnS2), whose band gap is about 1.9–2.4 eV, is one of the most important layered transition metal sulfides [35-37]. The unique atomic arrangement in SnS2, two layers of S atoms in the middle of two Sn atomic layers formed a built-in electric field perpendicular to the (001) crystal plane, is help to promote the directional migration of electrons [38-42]. And SnS2 as the n-type semiconductor was used to investigate the adjustment of electrons structure in photocatalytic system.
Herein, SnS2 nanosheets were in-situ constructed on the surface of 3DOM-SrTiO3, and novel SnS2/3DOM-SrTiO3 catalysts with Z-scheme heterojunction were obtained. SnS2 can absorb the full spectrum light and plays a role of electrons contributor. The photogenerated electrons achieved Z-scheme transmission affected by built-in electric field between layers via the hybrid interface [43-45]. SnS2/3DOM-SrTiO3 catalysts exhibited high the activity for CO2 photoreduction and altered the predominant products selectivity from CO to CH4 under full spectrum light irradiation in comparison to 3DOM-SrTiO3. Thus, the present work will propose a way for increasing multi-electrons reduction process and reach to the high yield of the multi-electron products of CO2 photoreduction.
The 3DOM-SrTiO3 was synthesized by colloidal crystal template (CCT) method and SnS2 was induced by co-hydrothermal as shown in Scheme S1 (Supporting information). The (SnS2)n/3DOM-SrTiO3 catalysts were obtained and the specific amount of reagent nd corresponding products are listed in Table S1 (Supporting information). For clarification of the prepared catalysts, Fig. 1 exhibits scanning electron microscopy (SEM) images of the catalysts. SnS2 shows regular hexagonal nanosheet structure with diameter of ~200 nm in Fig. S1a (Supporting information). As shown in Figs. 1b and c, the catalysts exhibit the ordered 3D network structure connected by the porous windows, and SnS2 nanosheets distribute to the ordered channels of 3DOM-SrTiO3. The compact arrangement structure benefits to the photoinduced electrons transfer between SnS2 and 3DOM-SrTiO3, and the relatively high specific surface area provides the abundant sites for CO2 adsorption and photoreduction in Table S2 (Supporting information). The X-ray diffraction (XRD) patterns were exhibited in Fig. S1 (Supporting information). The diffraction peaks of (SnS2)n/3DOM-SrTiO3 catalysts perfectly matched with Hexagonal phase SnS2 (JCPDS No. 23-0677) and Cubic phase SrTiO3 (JCPDS No. 35-0734), respectively.

|
Download:
|
| Fig. 1. SEM images of SnS2 (a), 3DOM-SrTiO3 (b) and (SnS2)3/3DOM-SrTiO3 (c) catalysts. | |
Furthermore, the phase structures of SnS2 and (SnS2)3/3DOM-SrTiO3 catalysts were further analyzed by Raman spectroscopy using an excitation wavelength of 532 nm (Fig. S2 in Supporting information). The Raman spectrum of SnS2 exhibits a strong band at 314 cm-1, which corresponds to the A1g vibration mode of SnS2. Moreover, a weak vibration peak at 205 cm-1 is shown in the inset of Fig. S2, it is attributed to the Eg vibration mode of SnS2. Meanwhile, the strong Raman bands of (SnS2)3/3DOM-SrTiO3 catalyst located at 145, 397, 517 and 638 cm-1 are attributed to the Raman-active transverse optical phonons of TO2, TO3, TO4 and longitudinal optical phonons of LO4 of SrTiO3, respectively. In addition, the A1g vibration peak of SnS2 detected at 314 cm-1. It demonstrates that the original chemical bond structure of the catalyst SrTiO3 support is not affected by the composite SnS2 nanosheets, and the appearance of the A1g vibration peak of SnS2 also indicates that it is tight contact with SrTiO3, resulting in heterojunction structure.
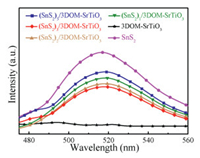
The light absorption property of the catalysts is precondition of well photocatalytic activity, the photoexcitation efficiency of charge separation depends on the light absorption efficiency. As shown in Fig. S3a (Supporting information), after SnS2 nanosheets constructed on 3DOM-SrTiO3, the optical absorption bands of (SnS2)n/3DOM-SrTiO3 catalysts have red-shift compared with pristine SrTiO3, indicating that the light absorption is enhanced in the visible region (from 400 nm to 800 nm). The photoluminescence (PL) spectra of the catalysts were used to provide evidences for further understand the photoinduced charge carrier migration and separation efficiency. Fig. 2 shows the PL spectrum of (SnS2)n/3DOM-SrTiO3 catalysts excited at an wavelength of 390 nm. Obviously, there is one PL emission peaks of pristine SnS2 located at 520 nm. It attributes to the recombination of electronhole pairs on the surface or inner of SnS2 nanosheets. However, 3DOM-SrTiO3 catalyst has not PL emission peak, indicating the low recombination. (SnS2)n/3DOM-SrTiO3 catalysts possess one PL emission peaks near to 520 nm, furthermore, the intensity of emission peak decreases with increasing of the SnS2 dosage, suggesting that the rapidly transfer of photoexcited electrons under irradiation could be suppressed. For the reason that the ECB and EVB of SnS2 are between ECB and EVB of SrTiO3, if carriers transfer through heterojunction Type Ⅰ path, they will rapidly recombine and fail to effective separation. So that electrons were located on SrTiO3 while the holes on SnS2 after separation and Z-scheme transmission. It is attributed to the Z-scheme heterojunction role of (SnS2)n/3DOM-SrTiO3 catalysts. It is noticed that, when SnS2 composite content is increased to 5%, the intensity of emission peak belongs to (SnS2)5/3DOM-SrTiO3 is enhanced. It indicates that the suitable dosage of SnS2 contributes to the charge transfer, and the excess SnS2 leads to the formation of recombination center, hence the photogenerated electron-hole pairs recombination efficiency increased.
|
Download:
|
| Fig. 2. The photoluminescence spectra of SnS2, 3DOM-SrTiO3 and (SnS2)n/3DOM-SrTiO3 catalysts. | |
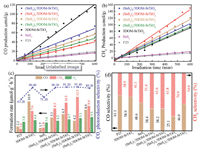
Photocatalytic CO2 reduction tests were conducted to evaluate the activity of the catalysts. The carbon-containing organic compounds of CO and CH4 as the predominant reduction products were detected, and their curves of output over time are shown in Figs. 3a and b, respectively. All the catalysts possess the activity for CO2 photoreduction as well as clean-energy production under the accordant conditions. 3DOM-SrTiO3 catalyst exhibits the largest formation rates of CO product, while (SnS2)3/3DOM-SrTiO3 catalyst has the largest formation rates of CH4 product. Actually, there are two reactions (CO2 reduction and H2O oxidation) in the whole photocatalysis system. The two reduction reactions require to consume photogenerated electrons. Hence, the photocatalytic reduction of H2O to H2 occurs as a competitive reaction to the CO2 photocatalytic reduction. In detail, 3DOM-SrTiO3 catalyst exhibits the higher selectivity (90.4%) for CO2 photoreduction in comparison with P25 (67.2%) and SnS2 (71.2%) shown in Fig. 3c. It contributes to the three-dimensional ordered macropores structure in favor of CO2 adsorption. Both P25 and 3DOM-SrTiO3 catalysts have the higher CO selectivity (Fig. 3d), which are 61.1% and 58.9%, respectively. It is attributed to the wide band gap limited the electron-hole pairs separation and hindered the multielectrons reduction process. In contrary, the pristine SnS2 catalyst possesses the narrow band gap performance with slightly higher CH4 selectivity (51.4%). After introduction of SnS2 nanosheets with narrow band gap, it is noted that the yield of CH4 product is enhanced while CO product is decreased (Fig. 3d). It suggests that the SnS2 nanosheets are able to act as electrons supplier, providing enough electrons for CO2 reduction as well as CH4 production. With the increasing dosage of SnS2, the activity of (SnS2)n/3DOM-SrTiO3 photocatalysts present a volcano variation trend. As shown in Table S3 (Supporting information), the yield of CH4 over (SnS2)n/3DOM-SrTiO3 catalysts reached to the highest amount about 12.5 μmol g-1 h-1 when the SnS2 dosage is 3.0%. In addition, (SnS2)3/3DOM-SrTiO3 catalyst also exhibits the best CO2 reduction property (91.4%) as well as CH4 selectivity (74.9%). The significant improvement of CH4 product selectivity can be affected by the binary compounds, indicating that SnS2 nanosheets play a key role to provide electrons in this photocatalytic CO2 reduction system. It is noteworthy that the binary catalysts consisted of the SnS2 and 3DOM-SrTiO3 inherited the preeminent CO2 photoreduction selectivity from 3DOM-SrTiO3. The formed Z-scheme heterojunction effectively increased the photogenerated electrons and greatly facilitates the enhancing selectivity and yield of CH4 product. The photocatalytic activity for CO2 reduction decreased with further increasing the amount of SnS2, indicating that the proportion of SnS2 in 3DOM-SrTiO3 is also crucial, the excess SnS2 possesses negative effect to the photocatalytic reaction, which is attributed to the direct recombination of photogenerated electron-hole pairs in the SnS2 nanosheets for decreasing photocatalytic performance of CO2 conversion. Although SnS2 can absorb the ultraviolet and visible light, the rapid recombination of photogenerated electronhole pairs limited its performance for CO2 photoreduction. It is consistent with the results of UV–vis and PL spectra. In addition, the half reaction of H2O oxidation to O2 caused by valence band of semiconductor is also significant. Therefore, the produced protons participate in the hydrogenation reactions for the formation of hydrocarbons.
|
Download:
|
| Fig. 3. CO (a) and CH4 (b) products over SnS2, 3DOM-SrTiO3 and (SnS2)n/3DOM-SrTiO3 catalysts. (c) CO2 photoreduction selectivity as well as the products formation rate of CO, CH4 and H2. (d) The CO and CH4 selectivity. | |
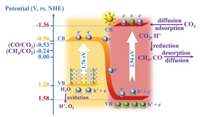
To understand the process of charge separation as well as CO2 photoreduction in this binary system, the pathway of electrons transmission and reactant conversion were proposed. As describes above, the energy gap (Eg) values of SrTiO3 and SnS2 are 2.94 eV and 1.76 eV, respectively (Fig. S3b in Supporting information). As shown in Fig. 4, the conduction band potential (ECB) of SrTiO3 (-1.36 V vs. NHE at pH 7) [46] and SnS2 (-0.56 V vs. NHE at pH 7) [47] is more negative than the reduction potentials of CO2 to CH4 (-0.24 V) and CO2 to CO (-0.53 V), hence both SrTiO3 and SnS2 could reduce CO2 to CH4 and CO in theory, which is consistent with the results of CO2 photoreduction. Moreover, the adsorption capacity of CO2 was enhanced by constructing the threedimensional ordered macropores structure in the present work.The perovskite type SrTiO3 catalyst containing alkalis was employed, for the interaction built between Sr and CO2, and which plays a significant role in the adsorption and activation of CO2 [48]. In order to improve the photon capture efficiency, the constructed 3DOM structure with unique pores which could appear the slow light effect [49], as well as the introduced SnS2 cocatalyst with narrow band gap is contributed to the excellent light adsorption. In addition, the separation and transfer efficiency of photogenerated carriers were enhanced by establishing Z-scheme heterojunction between SrTiO3 and SnS2.
|
Download:
|
| Fig. 4. Schematic diagram of electron-hole pairs separation and the possible reaction mechanism of SnS2/3DOM-SrTiO3 under simulative solar irradiation. | |
Herein, the binary (SnS2)n/3DOM-SrTiO3 catalysts exhibit higher performance for photocatalytic CO2 reduction than the SnS2 and 3DOM-SrTiO3 catalysts. Furthermore, the exist of SnS2 in the binary structure (SnS2/3DOM-SrTiO3) absolutely enhanced the CH4 selectivity compared with 3DOM-SrTiO3, especially altered the main selectivity from CO (SCO = 58.9% over 3DOM-SrTiO3) to CH4 (SCH4 = 74.9% over (SnS2)3/3DOM-SrTiO3). The SnS2 with the narrow band gap contributed to the large absorption in visible light region, resulting in the efficiency utilization of photons and more free electrons to be excited. The electrons separate with holes at the valence band of SnS2 and transfer to the conduction band, then transmit through the interface (between SnS2 and 3DOM-SrTiO3) to the conduction band of SrTiO3 which effected by the internal electric field of SnS2. Meanwhile, SrTiO3 excited by ultraviolet light and the photogenerated electrons transfer from valence band to conduction band. It demonstrates that the Z-scheme transmission of photoexcited electrons enabled the electron-hole pairs recombination decreased and enriched the high energy electrons at the conduction band of SrTiO3. In addition, the alkalis strontium on the surface of SrTiO3 contributes to the adsorption of CO2, resulting in the adequate adsorption intensity for CO2 accepting multielectrons. Thus, the multi-electrons process of CO2 reduction occurred, and the enhanced CH4 selectivity due to the localization of electrons and the suitable CO2 adsorption ability on the surface of SrTiO3. The binary compounds possess the higher performance for CO2 reduction and more multi-electrons process to form CH4.
In summary, the present work proposed a novel binary SnS2/3DOM-SrTiO3 with Z-scheme heterojunction improved the photocatalytic carbon dioxide reduction performance. The threedimensional ordered macropores structure of 3DOM-SrTiO3 facilitated the utilization of light, and the in situ introduced SnS2 with narrow band gap is benefits to the boosting absorption of visible light. The atom Sr as the alkalis in the perovskite type SrTiO3 is favor the adsorption and excitation of CO2. In addition, the Z-scheme transmission of photoexcited electrons enabled the electron-hole pairs recombination decreased and enriched the high energy electrons at the conduction band of SrTiO3, resulting the higher carbon dioxide reduction performance and more multi-electrons process to form CH4, hence the predominant products selectivity altered from CO to CH4. The study in this binary system provides a novel in-situ construction method and photogenerated electrons transfer strategy, the work may shed new light on the photoreduction performance and multi-electrons process improvement.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21673142, 21972166), Beijing Natural Science Foundation (No. 2202045), PetroChina Innovation Foundation (No. 2018D-5007-0505) and Science Foundation of China University of Petroleum, Beijing (Nos. 242017QNXZ02, 2462018BJC005).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.019.
| [1] |
Y. Yang, S. Ajmal, X. Zheng, Susrain. Energ. Fules 2 (2018) 510-537. |
| [2] |
S. Habisreutinger, L. Schmidt-Mende, J. Stolarczyk, Angew. Chem. Int. Ed. 52 (2013) 7372-7408. DOI:10.1002/anie.201207199 |
| [3] |
J.G. Ran, M. Jaroniec, S.Z. Qiao, Adv. Mater. 30 (2018) 1704649. DOI:10.1002/adma.201704649 |
| [4] |
A. Tjandra, J. Huang, Chin. Chem. Lett. 29 (2018) 734-746. DOI:10.1016/j.cclet.2018.03.017 |
| [5] |
C. Dong, J. Ji, Z. Yang, et al., Chin. Chem. Lett. 30 (2019) 853-862. DOI:10.1016/j.cclet.2019.03.020 |
| [6] |
L. Hao, L. Kang, H.W. Huang, et al., Adv, Mater. 31 (2019) 1900546. DOI:10.1002/adma.201900546 |
| [7] |
X. Chang, T. Wang, J. Gong, Energ. Environ. Sci. 9 (2016) 2177-2196. DOI:10.1039/C6EE00383D |
| [8] |
L. Qiao, M. Song, A. Geng, et al., Chin. Chem. Lett. 30 (2019) 1273-1276. DOI:10.1016/j.cclet.2019.01.024 |
| [9] |
C. Wang, Y. Zhao, H. Xu, et al., Appl. Catal. B 263 (2020) 118314. DOI:10.1016/j.apcatb.2019.118314 |
| [10] |
F. Wang, X. Yu, M. Ge, et al., Chem. Eng. J. 384 (2020) 123381. DOI:10.1016/j.cej.2019.123381 |
| [11] |
S. George, S. Pokhrel, Z. Ji, et al., J. Am. Chem. Soc. 133 (2011) 11270-11278. DOI:10.1021/ja202836s |
| [12] |
T. Inoue, A. Fujishima, S. Konishi, et al., Nature 277 (1979) 637-638. DOI:10.1038/277637a0 |
| [13] |
H.J. Yoon, M.S.K. Kim, W.X. Huang, et al., Chin. Chem. Lett. 29 (2018) 800-804. DOI:10.1016/j.cclet.2018.01.021 |
| [14] |
J. Shan, F. Raziq, M. Humayun, et al., Appl. Catal. B 219 (2017) 10-17. DOI:10.1016/j.apcatb.2017.07.024 |
| [15] |
K. Shao, Y. Wang, M. Iqbal, et al., Appl. Surf. Sci. 434 (2018) 717-724. DOI:10.1016/j.apsusc.2017.11.004 |
| [16] |
C. Luo, J. Zhao, Y. Li, et al., Appl. Surf. Sci. 447 (2018) 627-635. DOI:10.1016/j.apsusc.2018.04.049 |
| [17] |
Q. Kang, T. Wang, P. Li, et al., Angew. Chem. Int. Ed. 54 (2015) 841-845. DOI:10.1002/anie.201409183 |
| [18] |
S. Shoji, A. Yamaguchi, E. Sakai, et al., ACS Appl. Mater. Interfaces 9 (2017) 20613-20619. DOI:10.1021/acsami.7b05197 |
| [19] |
T. Townsend, N. Browning, F. Osterloh, ACS Nano 6 (2012) 7420-7426. DOI:10.1021/nn302647u |
| [20] |
J. Lan, D.P. Cao, W.C. Wang, et al., ACS Nano 4 (2010) 4225-4237. DOI:10.1021/nn100962r |
| [21] |
X. Meng, S. Ouyang, T. Kako, et al., Chem. Commun. 50 (2014) 11517-11519. DOI:10.1039/C4CC04848B |
| [22] |
Q. Tang, Z. Sun, P. Wang, et al., Appl. Surf. Sci. 463 (2019) 456-462. DOI:10.1016/j.apsusc.2018.08.245 |
| [23] |
S. Shoji, G. Yin, M. Nishikawa, et al., Chem. Phys. Lett. 658 (2016) 309-314. DOI:10.1016/j.cplett.2016.06.062 |
| [24] |
B. Kumar, J. Smieja, C. Kubiak, J. Phys. Chem. C 114 (2010) 14220-14223. DOI:10.1021/jp105171b |
| [25] |
S. Xie, J. Deng, S. Zang, J. Catal. 322 (2015) 38-48. DOI:10.1016/j.jcat.2014.09.024 |
| [26] |
J. Liang, Y. Zheng, J. Chen, et al., Angew. Chem. Int. Ed. 51 (2012) 3892-3896. DOI:10.1002/anie.201107981 |
| [27] |
M. Zalfani, B. Schueren, M. Mahdouani, et al., Appl. Catal. B 199 (2016) 187-198. DOI:10.1016/j.apcatb.2016.06.016 |
| [28] |
Y. Chang, K. Yu, C.X. Zhang, et al., Appl. Catal. B 215 (2017) 74-84. DOI:10.1016/j.apcatb.2017.05.054 |
| [29] |
J. Liu, H. Zhao, M. Wu, et al., Adv. Mater. 29 (2017) 1605349. DOI:10.1002/adma.201605349 |
| [30] |
Y. Lu, H. Yu, S. Chen, et al., Environ. Sci. Technol. 46 (2012) 1724-1730. DOI:10.1021/es202669y |
| [31] |
L. Sun, M. Yang, J. Huang, et al., Adv. Funct. Mater. 26 (2016) 4943-4950. DOI:10.1002/adfm.201600894 |
| [32] |
Q. Wu, M. Jing, Y. Wei, et al., Appl. Catal. B:Environ. 244 (2019) 628-640. DOI:10.1016/j.apcatb.2018.11.094 |
| [33] |
L. Tang, Z. Zhao, Y.C. Wei, et al., Catal. Today 297 (2017) 131-142. DOI:10.1016/j.cattod.2017.06.016 |
| [34] |
Y. Wei, J. Liu, Z. Zhao, et al., Energ. Environ. Sci. 4 (2011) 2959-2970. DOI:10.1039/c0ee00813c |
| [35] |
Y. Zhang, Z. Du, K. Li, et al., ACS. Appl. Mater. Interfaces 3 (2011) 1528-1537. DOI:10.1021/am200102y |
| [36] |
Z. Zhang, J. Huang, M. Zhang, et al., Appl. Catal. B 163 (2015) 298-305. DOI:10.1016/j.apcatb.2014.08.013 |
| [37] |
J. Yu, C. Xu, F. Ma, et al., ACS. Appl. Mater. Interfaces 6 (2014) 22370-22377. DOI:10.1021/am506396z |
| [38] |
S. Chen, J. Wang, J. Huang, et al., Chin. J. Chem. Phys. 30 (2017) 36-42. DOI:10.1063/1674-0068/30/cjcp1605113 |
| [39] |
J. Liu, E. Hua, J. Phys, Chem. C 121 (2017) 25827-25835. |
| [40] |
Y. Wang, Y. Tian, Z. Lang, et al., J. Mater. Chem. A 6 (2018) 21056-21063. DOI:10.1039/C8TA07352J |
| [41] |
Y. Ma, X. Zhao, M. Niu, et al., RSC Adv. 7 (2017) 25582-25588. DOI:10.1039/C7RA01920C |
| [42] |
H.M. Huang, B.Y. Dai, W. Wang, et al., Nano Lett. 17 (2017) 3803-3808. DOI:10.1021/acs.nanolett.7b01147 |
| [43] |
Y. Li, K. Lv, W. Ho, et al., Int. J. Appl. Catal. B 202 (2017) 611-619. DOI:10.1016/j.apcatb.2016.09.055 |
| [44] |
W. Ouyang, F. Teng, X. Fang, Adv. Func. Mater. 28 (2018) 1707178. DOI:10.1002/adfm.201707178 |
| [45] |
M. Ding, J. Zhou, H. Yang, et al., Chin. Chem. Lett. 31 (2020) 71-76. DOI:10.1016/j.cclet.2019.05.029 |
| [46] |
H. Tan, Z. Zhao, W. Zhu, et al., ACS Appl. Mater. Interfaces 6 (2014) 19184-19190. DOI:10.1021/am5051907 |
| [47] |
T. Di, B. Zhu, B. Cheng, et al., J. Catal. 352 (2017) 532-541. DOI:10.1016/j.jcat.2017.06.006 |
| [48] |
X. Wu, C. Wang, Y. Wei, et al., J. Catal. 377 (2019) 309-321. DOI:10.1016/j.jcat.2019.07.037 |
| [49] |
Y. Wei, J. Jiao, Z. Zhao, et al., Appl. Catal. B 179 (2015) 422-432. DOI:10.1016/j.apcatb.2015.05.041 |
 2020, Vol. 31
2020, Vol. 31 

