b Engineering Research Center for Waste Oil Recovery Technology and Equipment, Ministry of Education, Chongqing Technology and Business University, Chongqing 400067, China;
c Chongqing Key Laboratory of Three Gorges Reservoir Area Surface Process and Environmental Remote Sensing, Chongqing Key Laboratory of Inorganic Functional Materials, Chongqing Normal University, Chongqing 401331, China
In recent years, numerous efforts have been focused on the improvement of catalysts based on palladium nanoparticles (Pd NPs) for electrocatalytic hydrodechlorination (EHDC) reaction [1-4]. For typical probe EHDC reaction towards 2, 4-dichlorophenol (2, 4-DCP), the electronic structure of Pd directly affects the preference of H adsorbed on surface (H*) to participate in hydrogen evolution reaction (HER) or EHDC reaction [5]. In general, the optimal HER reaction corresponds to a H-adsorption Gibbs free energy of zero [6, 7]. The H adsorption strength is further enhanced to be more beneficial to the EHDC than to the HER [8]. Similarly, enhancing the competitive adsorption of 2, 4-DCP at the Pd sites in the reaction system (including the possible intermediate monochlorophenol or the product phenol) is expected to promote dechlorination efficiency. Many attempts were thus made to rationally design bimetallic [4, 5, 9, 10] and trimetallic nanoparticles [11, 12] based on a single Pd component to improve the electrocatalytic activity, with controlled sizes [12], core-shell structures [5], and/or optimized content of alloying metals [13]. However, the fabrication processes are rather complicated, which limits their practical applications. Metal-support hybrid materials have aroused practical interest owing to their wide applications in heterogeneous catalysis [14, 15]. The surface properties and spatial structure of the support perform remarkable functions in promoting the selectivity of a reaction, whereas proper metal-support interaction and good dispersion of the metal nanoparticles cause the high catalytic performance and selectivity via an electronic effect [16-21]. Therefore, it is potential to improve the EHDC efficiency of 2, 4-DCP by modifying the characteristics of Pd NPs through metal-support effect.
Polymeric carbon nitrides (PCN) are easily synthesized from abundant and inexpensive raw materials, and previous studies have shown that PCN is indeed an effective support for metal dispersion with abundant nitrogen sites, which can serve as active sites to anchor metal particle [15, 17, 22-25]. The strong interaction between metal and PCN can be exploited to improve the catalytic activity and stability of the metal NPs in catalytic reactions [18, 26-30]. More importantly, the properties of PCN materials can be easily optimized by doping with heteroatoms [31-34], coupling with other semiconductors or conductors [35, 36], as well as introducing defects [37-39]. Recently, reports [40, 41] of one-step KOH-assisted method to prepare defective (cyano, -C≡N) PCN have attracted our attention. According to our preliminary studies [42-44], Pd NPs in an electron-rich state are favorable for EHDC reactions. Theoretically, -C≡N group as a strong electron acceptor is expected to serve as a bridge for electron transfer from PCN to Pd NPs, further enhancing the EHDC efficiency of Pd NPs.
Herein, a simple pyrolysis route in the presence of KOH is employed to fabricate defective PCN (PCN-x) [45], which is subsequently used as a modified support for supporting Pd NPs by a wet chemical method. The structure, composition and electronic interaction of the as-prepared Pd/PCN-x series catalysts were systematically characterized. And then the working electrode referred to as a Pd/PCN-x electrode was prepared, corresponding to the Pd/PCN-x catalyst. The catalytic activity of the obtained electrode was examined by using 2, 4-DCP to P as a model reaction for liquid phase electrocatalytic hydrodechlorination at room temperature. (The detailed experimental content is given in the Supporting information).
X-ray diffraction (XRD) patterns in Fig. S1a (Supporting information) demonstrate that the as-synthesized metallic Pd is of a face-centered-cubic crystal structure in all the Pd/PCN-x samples. The diffraction peaks at 2θ = 39.4°, 45.8°, and 66.8° can be assigned to the (111), (200) and (220) crystal planes of Pd (JCPDS No. 89-4897), respectively [16, 46]. The additional two peaks at 2θ = 12.8° and 26.7° are the characteristic diffraction peaks of PCN (Fig. S1b in Supporting information) [47, 48], the intensities of which considerably decrease after introduction of the defects and Pd NPs, evidencing a loss of the ordered structure within the PCN framework. The time-resolved photoluminescence (PL) spectra in Fig. S1c (Supporting information) reveal that the signals of PCN-0.05 and PCN-0.25 become weaker and red shift, in comparison to that of PCN. It can be attributed to the formation of defects in PCN-x by KOH etching, which has modified the band/electronic structure of PCN-x [45]. Furthermore, all the Pd/PCN-x delivers weaker PL signals than the corresponding PCN-x (Fig. S1d in Supporting information), indicating the successful deposition of Pd NPs, which impedes the recombination of photogenerated electron-hole pairs [48, 49]. Figs. S2a and S2b (Supporting information) present the transmission electron microscope (TEM) images of Pd/PCN-0 and Pd/PCN-0.05, and the NPs are well-dispersed on the support surface with diameters concentrated at about 5 nm. The high resolution TEM (HRTEM) images in Figs. S2c and S2d (Supporting information) display a characteristic lattice spacing of ~0.23 nm on the synthesized NPs, which is well matched with the (111) plane of the metallic Pd [2, 3].
The Fourier transform infrared spectroscopy (FTIR) spectra of the pristine PCN-x are demonstrated in Fig. S3a (Supporting information). The broad peak between 3000 and 3650 cm-1 is ascribed to N-Hx or O-H stretching, and more specifically, the peak concentrated at 3200 cm-1 belongs to N-Hx. The peaks in the range of 900 to 1800 cm-1 arise from the stretching mode of the N-C = N heterorings in the "melon" framework, whilst the peak at 810 cm-1 is attributed to the characteristic heptazine breathing pattern. It is worth noting that as the x increases, a new absorption peak appears at 2178 cm-1, corresponding to the asymmetric stretching vibration of the -C≡N groups [40, 41, 45]. It is inferred that the -C≡N groups are formed by the reaction of OH- released by KOH melting with the amine group of the urea-derived intermediate during thermal polymerization [45]. As shown in Fig. 1a, the absorption bands of all above groups are preserved after the deposition of Pd NPs, but those attributed to the -C≡N and heptazine groups decrease in the intensity, indicating the PCN-x might anchor the Pd NPs via the -C≡N and heptazine groups. Furthermore, a new peak appears in Pd/PCN-x at 620 cm-1 which is absent after treatment of PCN-x by NaBH4 alone (Figs. S3b and S3c in Supporting information) [50], demonstrating the formation of the Pd-C bond.

|
Download:
|
| Fig. 1. FTIR spectra (a), XPS spectra of a wide scan survey (b), and high-resolution XPS spectra of C 1s, N 1s and Pd 3d of Pd/PCN-x (c-e). | |
To further investigate the surface compositions of the catalyst and electronic interactions among the component elements, X-ray photoelectron spectroscopy (XPS) analyses on Pd/PCN-x were conducted. Fig. 1b shows a comparison in the survey spectra of bare and Pd-decorated PCN-x surfaces. All spectra show the strong core-level binding energy peaks for C 1s and N 1s, and a weak peak for O 1s. The presence of the Pd 3d and Pd 3p peaks confirm the successful formation of Pd NPs on PCN-x. No characteristic peak of K element is found for both PCN-x and Pd/PCN-x, indicating the K is not included in the Pd/PCN-x catalysts.
For the series of PCN-x supports, the C 1s XPS spectrum in Fig. 1c is deconvoluted into three peaks centered at 288.2, 286.4, and 284.8 eV, corresponding to N-C = N coordination in the framework of PCN, C-NHx (x = 1, 2) at the edge of heptazine units and the adventitious hydrocarbons, respectively [40, 41, 45]. Notably, the 286.4 eV C 1s signals of PCN-0.25 and PCN-0.50 are intensified compared to that of PCN-0, which can be taken as an additional evidence for the formation of -C≡N group (as seen by FTIR). The deconvoluted N 1s XPS spectrum is fitted into three peaks at around 398.7, 400.3, and 401.2 eV, corresponding to the bicoordinated (N2C) and tri-coordinated (N3C) nitrogen atoms as well as N-Hx groups in the heptazine framework [45]. As seen, with an increased x, the binding energy of the N3C peak shifts from 400.3 to 399.6 eV, owing to the formation of an increased amount of -C≡N groups (its N 1s binding energy is between that of N2C and N3C). In addition, as listed in Table S1 (Supporting information), the N/C atomic ratio obtained from the XPS data shows a progressive decrease from 1.32 to 0.80 as the dose of KOH increases, and the atomic ratio of N2C/N3C drops from 7.2 to 4.5, indicating the unique function of KOH in selectively etching the N from PCN network to introduce the N2C vacancies. On basis of the XPS results, a fine PCN and a defective PCN structure are schemed in Fig. S4 (Supporting information). According to the results in Fig. 1d, Figs. S3d and S3e and Tables S1 and S2 (Supporting information), after the Pd deposition on PCN-x, the N/C ratio and the relative content of N2C to N3C are further reduced, and the binding energy of N2C blueshifts from 398.7 to 398.9 eV. As the NaBH4 treatment for PCN does not change its structure (Fig. S3b), these changes should confirm the formation of Pd-N2C bond [51]. The high-resolution Pd 3d XPS spectra for all the Pd/PCN-x samples in Fig. 1e and Fig. S3f (Supporting information) display the typical doublets. A pair centered on 335.20 and 340.46 eV corresponds to the metallic Pd (0) state of Pd 3d5/2 and Pd 3d3/2, respectively [46, 52]. Another pair positioned at 336.70 and 341.96 eV is attributed to the Pd(Ⅱ) oxidation state, indicating that the Pd surface is partially oxidized. The ratio of Pd0/Pd2+ in Pd/PCN-0.05 is 2.3, higher than that of 2.0 in Pd/PCN-0 and Pd/PCN-0.25 samples. Noted the binding energy of Pd 3d varies little in spite of the formation of Pd-N/C bonds, which can be ascribed to the combined effect of different defects with Pd (as revealed by the DFT calculations below).
EHDC experiments performed on Pd/PCN-x electrodes show that the mass balance of the total carbon remains relatively constant during EHDC of 2, 4-DCP (Fig. 2a). The EHDC products are detected as most P and a small amount of 2-CP. As the EHDC progresses, the molar ratio of 2-CP to 2-CP + P shown in Fig. S5 (Supporting information) gradually decreases to less than 10% after 3 h of the reaction. Therefore, 2-CP and P are inferred as intermediate and final products, respectively. The EHDC performances of all the Pd/PCN-x are compared in Fig. 2b, and all of them are catalytically active for the removal of 2, 4-DCP. The EHDC reaction on Pd/PCN-0.10 proceeds the most effective, and its EHDC efficiency at 3 h reaches 67%, much higher than the 43%, 51%, 59%, 53% and 47% for Pd/PCN-0, Pd/PCN-0.01, Pd/PCN-0.05, Pd/PCN-0.25 and Pd/PCN-0.50, respectively. The kinetics follows the pseudo first-order rate law (R2 > 0.99), and the apparent rate constants (Kobs) are normalized (Knor) by electrochemically active surface area (see the determination process in Supporting information) to highlight the specific activity of the catalyst (Fig. S6 and Table S4 in Supporting information). The results in Fig. 2c show that the specific activity of Pd/PCN-x varies in a volcano-like trend with x, where Pd/PCN-0.05 delivers the largest one of 0.09 min-1 mPd-2. Therefore, the optimized interactions of Pd NPs with PCN-0.05 support play an important role in boosting the EHDC reaction. Furthermore, the results in Figs. S7 and S8 (Supporting information) show that both Pd/PCN-0 and Pd/PCN-0.05 electrodes have durable EHDC performance with little change in the timedependent 2, 4-DCP removal curve, and the Pd NPs preserve the size, dispersion, morphology and exposed crystal plane after five cycles of reaction. Fig. 2d and Fig. S9 (Supporting information) plots the current efficiency variation with the x. Obviously, Pd/PCN-0.05 and Pd/PCN-0.10 possess the highest current efficiency. However, the value is relatively below 40%, indicating a large waste of the energy. Current efficiency is a critical descriptor of the competing kinetics of EHDC and HER. Higher current efficiency suggests that EHDC is more prevalent than HER. Therefore, promoting the intermediate species H* to participate in EHDC reaction rather than generating H2 is considered as a promising way to improve EHDC efficiency.

|
Download:
|
| Fig. 2. Intermediates and final products during EHDC reaction (a) (hollow points represent the total mass balance), EHDC performance (b), normalized reaction rate constant Knor (inset is the corresponding linearized pseudo first-order kinetic profiles) (c), and current efficiency (d) for Pd/PCN-x electrode (2, 4-DCP0 ≈ 50 mg/L, applied potential = -0.8 V vs. Ag/AgCl, 50 mmol/L Na2SO4, initial pH 6.8). | |
Tert-butanol (TBA), a specific quencher for H*, was used to identify the effect of atomic H* on EHDC of 2, 4-DCP in Pd/PCN-0 and Pd/PCN-0.05 cathode systems [5]. As seen in Figs. 3a and b, the negative effect of TBA on the Pd/PCN-0 electrode is not significant. However, for the Pd/PCN-0.05 electrode, the EHDC reaction is gradually suppressed with the level of TBA increasing from 0 to 1.0 mmol/L. Specifically, in the presence of 0.5 mmol/L TBA, the EHDC efficiency of Pd/PCN-0.05 is reduced by 23%, while that of Pd/PCN-0 is almost unchanged. With the amount of TBA increasing to 1.0 mmol/L, the EHDC efficiency of Pd/PCN-0.05 and Pd/PCN-0 decreases by 27% and 12%, respectively. Overall, the EHDC performance of the Pd/PCN-0.05 electrode is more affected by the decrease in H* content, but its EHDC activity is higher, indicating that H* accessible for EHDC reactions on Pd/PCN-x series's electrodes is sufficient, and other reaction steps should therefore be the rate-determining step.

|
Download:
|
| Fig. 3. C/C0 in different cathode systems with various TBA concentrations (a), EHDC efficiency with various TBA concentrations (b), C/C0 in different cathode systems with P (Pre-saturation with 30 mmol/L P) (c), and EHDC efficiency and its decrease for 2, 4-DCP reduction of catalyst pre-saturated with 30 mmol/L P (d). | |
Our previous studies have shown that the adsorption behaviors of the reactant 2, 4-DCP and the product P on the surface of Pd/C are similar, including the optimal adsorption configuration and the adsorption energy [42, 43, 46]. The Pd based catalysts usually suffer from the phenol poisoning on the active sites. We then performed batch EHDC experiments to verify the effect of P pre-adsorption on our Pd/PCN-x electrodes. Figs. 3c and d show that the P adsorption on Pd/PCN-x surface is indeed detrimental to the EHDC reaction with the efficiency decreasing for all electrodes after the introduction of P at the beginning of reaction. Notably, the EHDC efficiency of Pd/PCN-0 saturated with P is reduced by as much as 40%, which is about twice the decrease in activity of other electrodes. This means that the stronger affinity of P with Pd/PCN-0 limits the adsorption, activation and reaction of 2, 4-DCP on the electrode surface. In contrast, the interaction between Pd and defective PCN-0.05 or PCN-0.10 alleviates the strong occupation of P at the Pd sites, allowing 2, 4-DCP to continuously adsorb on the catalyst to participate in the EHDC reaction. The decrease in EHDC efficiency exhibits a typical volcano curve, again emphasizing the optimal interaction of Pd NPs with PCN-x supports.
To obtain a deep understanding of the superior EHDC performance of Pd/PCN-x, a series of Density functional theory (DFT) calculations were performed to unravel the interactions between the metal and support, and how they alter the adsorption characteristics of H*, 2, 4-DCP and P on Pd NPs. Combined with the results of FTIR and XPS, we first constructed a PCN support with -C≡N defects, -Nv defects or both of them, on basis of the fact that the -C≡N defects are easy to form by KOH etching, but the -Nv defects are dominant in Pd/PCN-x prepared with a larger x. Next, the structure of the corresponding Pd-loaded PCN-x was optimized, and the Pd7 cluster was used to model the Pd NPs. As shown in Fig. S10 (Supporting information), Pd NPs can interact with PCN-x at the hollow site among tri-s-triazine units to form a twisted decahedral structure with Pd-N and Pd-C bonds.Quantitatively, the Bader charge analysis shows that the -C≡N group transfers electrons (-0.01|e|, to the Pd7 cluster, achieving electron enrichment on Pd, while the -Nv defect grabs about -0.4|e| from Pd NPs. This indicates that the -C≡N defect and the -Nv defect have an inverse effect on electronic structure of the Pd NPs at the DFT calculation level. In this case, the EHDC performance of Pd/PCN-x largely depends on species of the defects, the Pd NPs interact as well as their relative number.
In many cases, the Gibbs free-energy of the intermediate state |∆GH*|, is considered as a major descriptor of H* generation and HER kinetics on solid electrodes. Figs. 4a and b shows the Gibbs energy of H and the corresponding adsorption configuration adsorbed on the defective surface. The ∆GH1* value of H1* is negative and relatively close to 0 eV (the optimal value for HER), indicating that all surface has excellent H1* generation activity. Especially for Pd/PCN surfaces, HER is more preferable than other surfaces from the thermodynamics view of point [17, 18]. In the presence of H1*, the adsorption of H2 on different surfaces is enhanced in the order of Pd/PCN(-Nv) > Pd/PCN(-C≡N/-Nv) > Pd/PCN > Pd/PCN(-C≡N). On the surface of Pd/PCN(-Nv), H2* exhibits a |∆GH2*| value corresponding to the chemisorption characteristics, which is unfavorable for its subsequent reduction reaction. As the H* coverage increases, the adsorption of H3 is only promoted on the Pd/PCN surface and is weakened on other surfaces, especially on the surface containing -C≡N defects. It means that in comparison to Pd-PCN, the H3* generation on the other three surfaces would be relatively difficult, and the generated H* incline to evolve to molecular H2, leading to a lower H* coverage. These DFT calculations rationalize that Pd/PCN with excellent H* generation ability, appears to be less sensitive to TBA concentrations in the above H*-quenching experiments.
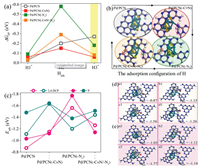
|
Download:
|
| Fig. 4. Free energy diagram of H adsorbed one by one on Pd/PCN surfaces with different defects (a), and corresponding H* configuration (b). The adsorption energies of 2, 4-DCP and P on different surfaces (c) (Rose red indicates that the adsorption site is adjacent to the cyano group, and green indicates that the adsorption site is adjacent to the nitrogen vacancy.); top view of the corresponding adsorption configurations of 2, 4-DCP (d) and P (e) (a: Pd/PCN; b: Pd/PCN(-C≡N); c: Pd/PCN(-Nv) and d: Pd/CPN(-C≡N/-Nv)). | |
To illustrate the effects of -C≡N and -Nv defects on the adsorption of 2, 4-DCP and P on Pd NPs, we performed a series of DFT calculations. The adsorption energy is depicted in Figs. 4c and d, and the corresponding adsorption configuration is given in Fig. S11 (Supporting information). The P binds much stronger with Pd/PCN than the 2, 4-DCP for the two selected adsorption configurations, and the corresponding binding energies are 1.48 and 1.11 eV, respectively. The preferential P adsorption on the Pd/PCN surface would hamper the effective adsorption of 2, 4-DCP for subsequent EHDC reaction [44], which is consistent with the observations in the P pre-adsorption experiment (Fig. 3). When the surface of Pd/PCN-x contains single -C≡N defect or single -Nv defect, the relative adsorption strength of 2, 4-DCP is increased. Especially on the surface where -C≡N group exists, the adsorption energy of 2, 4-DCP is close to P, indicating that Pd/PCN(-C≡N) shows enhanced tolerance to the Pd site blocking by P. Interestingly, P, but not 2, 4-DCP, preferentially adsorbs on the Nv-regulated Pd/PCN(-Nv) surface, leading to the inactivation of Pd sites, which explains the significant decrease in EHDC activity on Pd/PCN-0.50. Thus, the interaction of Pd with the supports can mediate the adsorption-desorption behavior of 2, 4-DCP and P to promote the EHDC reaction. Specifically, the interactions of Pd with the -C≡N group promote the adsorption of 2, 4-DCP, while inversely, those with -Nv defects facilitate the adsorption of P. Accordingly, the climbing part of the volcano curve for the EHDC efficiency can be attributed to the dominative number of the -C≡N defects in the sample, while the declining part is ascribed to the increase in the number of -Nv defect in the sample.
Our study indicates that optimizing the structure of the PCN support results in the formation of a simple, robust and efficient Pd/PCN-x hybrid catalyst with improved EHDC performance. Experimental observations combined with DFT calculations reveal that the strong adsorption of P blocks the active site on the surface of Pd/PCN, which severely limits its dechlorination performance. PCN-x containing -C≡N defects effectively mediates the spatial configuration and electronic structure of Pd, and promotes the preferential adsorption of 2, 4-DCP rather than P, resulting in a smooth EHDC reaction.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe present work is financially supported by the National Natural Science Foundation of China (Nos. 51878105 and 51978110), Innovation group of New Technologies for Industrial pollution control of chongqing education commission (CXQT19023), Scientific and Technological Research Program of Chongqing Municipal Education Commission (Nos. KJQN201800829, KJZD-K201900502, KJZD-M201900802 and KJZD-K201800801).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.022.
| [1] |
X. Ding, Z. Yao, Y. Xu, et al., J. Catal. 368 (2018) 336-344. DOI:10.1016/j.jcat.2018.10.008 |
| [2] |
R. Mao, H. Lan, L. Yan, et al., Environ. Sci-Nano 5 (2018) 2282-2292. DOI:10.1039/C8EN00727F |
| [3] |
X. Shu, Q. Yang, F. Yao, et al., Chem. Eng. J. 358 (2019) 903-911. DOI:10.1016/j.cej.2018.10.095 |
| [4] |
J. Zhou, Z. Lou, K. Yang, et al., Appl. Catal. B-Environ. 244 (2019) 215-224. DOI:10.1016/j.apcatb.2018.11.052 |
| [5] |
R. Mao, C. Huang, X. Zhao, M. Ma, J. Qu, Appl. Catal. B-Environ. 241 (2019) 120-129. DOI:10.1016/j.apcatb.2018.09.013 |
| [6] |
E. Skúlason, V. Tripkovic, M.E. Bjǒrketun, et al., J. Phys. Chem. C 114 (2010) 18182-18197. DOI:10.1021/jp1048887 |
| [7] |
Z. Zhuang, Y. Li, Z. Li, et al., Angew. Chem. Int. Ed. 57 (2018) 496-500. DOI:10.1002/anie.201708748 |
| [8] |
I. López-Corral, E. Germán, A. Juan, M.A. Volpe, G.P. Brizuela, J. Phys, Chem. C 115 (2011) 4315-4323. |
| [9] |
L. Yang, Z. Chen, D. Cui, et al., Chem. Eng. J. 359 (2019) 894-901. DOI:10.1016/j.cej.2018.11.099 |
| [10] |
R. Liu, H.M. Chen, L.P. Fang, et al., Environ. Sci. Technol. 52 (2018) 4244-4255. DOI:10.1021/acs.est.7b05996 |
| [11] |
Z. Lou, J. Zhou, M. Sun, et al., Chem. Eng. J. 352 (2018) 549-557. DOI:10.1016/j.cej.2018.07.057 |
| [12] |
R. Liu, H. Zhao, X. Zhao, et al., Environ. Sci. Technol. 52 (2018) 9992-10002. DOI:10.1021/acs.est.8b02740 |
| [13] |
D. Xu, L. Zhang, H. Wang, Z. Bian, Chem. Eng. J. 358 (2019) 1371-1382. DOI:10.1016/j.cej.2018.10.129 |
| [14] |
F. Wang, B. Tu, P. He, et al., Adv. Mater. 31 (2019) 1805317. DOI:10.1002/adma.201805317 |
| [15] |
Y.Y. Cai, X.H. Li, Y.N. Zhang, et al., Angew. Chem. Int. Ed. 52 (2013) 11822-11825. DOI:10.1002/anie.201304652 |
| [16] |
L. Cao, Q. Luo, W. Liu, et al., Nat. Catal. 2 (2018) 134-141. |
| [17] |
S. Cao, H. Li, T. Tong, et al., Adv. Funct. Mater. 28 (2018) 1802169. DOI:10.1002/adfm.201802169 |
| [18] |
L. Zhang, F. Mao, L.R. Zheng, et al., ACS Catal. 8 (2018) 11035-11041. DOI:10.1021/acscatal.8b03789 |
| [19] |
A. Mitra, P. Howli, D. Sen, B. Das, K.K. Chattopadhyay, Nanoscale 8 (2016) 19099-19109. DOI:10.1039/C6NR06837E |
| [20] |
Y.N. Chen, G.C. Egan, J.Y. Wan, et al., Nat. Commun. 7 (2016) 12332. DOI:10.1038/ncomms12332 |
| [21] |
Z.L. Chen, Y.W. Liu, C. Liu, et al., Small 16 (2020) 1904964. DOI:10.1002/smll.201904964 |
| [22] |
Y. Li, X. Xu, P. Zhang, et al., RSC Adv. 3 (2013) 10973-10982. DOI:10.1039/c3ra41397g |
| [23] |
J.H. Lee, J. Ryu, J.Y. Kim, et al., J. Mater. Chem. A 2 (2014) 9490-9495. DOI:10.1039/c4ta01133c |
| [24] |
P. Fageria, S. Uppala, R. Nazir, et al., Langmuir 32 (2016) 10054-10064. DOI:10.1021/acs.langmuir.6b02375 |
| [25] |
X. Chen, X. Zhao, Z. Kong, W.J. Ong, N. Li, J. Mater, Chem. A 6 (2018) 21941-21948. |
| [26] |
X. Shao, J. Xu, Y. Huang, et al., AIChE J. 62 (2016) 2410-2418. DOI:10.1002/aic.15218 |
| [27] |
S. Cao, Y. Li, B. Zhu, M. Jaroniec, J. Yu, J. Catal. 349 (2017) 208-217. DOI:10.1016/j.jcat.2017.02.005 |
| [28] |
T.K. Das, S. Banerjee, B. Vishwanadh, R. Joshi, V. Sudarsan, Solid State Sci. 83 (2018) 70-75. DOI:10.1016/j.solidstatesciences.2018.06.011 |
| [29] |
T. Tong, B. Zhu, C. Jiang, B. Cheng, J. Yu, Appl. Surf. Sci. 433 (2018) 1175-1183. DOI:10.1016/j.apsusc.2017.10.120 |
| [30] |
K. Gu, X.T. Pan, W.W. Wang, et al., Small 14 (2018) 1801812. DOI:10.1002/smll.201801812 |
| [31] |
S.C. Yan, Z.S. Li, Z.G. Zou, Langmuir 26 (2010) 3894-3901. DOI:10.1021/la904023j |
| [32] |
J. Li, B. Shen, Z. Hong, et al., Chem. Commun. 48 (2012) 12017-12019. DOI:10.1039/c2cc35862j |
| [33] |
G. Liu, P. Niu, C.H. Sun, et al., J. Am. Chem. Soc. 132 (2010) 11642-11648. DOI:10.1021/ja103798k |
| [34] |
J.R. Ran, T.Y. Ma, G.P. Gao, X.W. Du, S.Z. Qiao, Energ. Environ. Sci. 8 (2015) 3708-3717. DOI:10.1039/C5EE02650D |
| [35] |
L. Ge, C. Han, J. Liu, Appl. Catal. B-Environ. 108 (2011) 100-107. |
| [36] |
A. Du, S. Sanvito, Z. Li, et al., J. Am. Chem. Soc. 134 (2012) 4393-4397. DOI:10.1021/ja211637p |
| [37] |
P. Niu, G. Liu, H.M. Cheng, J. Phys. Chem. C 116 (2012) 11013-11018. DOI:10.1021/jp301026y |
| [38] |
Z. Hong, B. Shen, Y. Chen, et al., J. Mater. Chem. A 1 (2013) 11754-11761. DOI:10.1039/c3ta12332d |
| [39] |
P. Niu, L.C. Yin, Y.Q. Yang, G. Liu, H.M. Cheng, Adv. Mater. 26 (2014) 8046-8052. DOI:10.1002/adma.201404057 |
| [40] |
Y. Wu, Q. Chen, S. Liu, et al., Chin. Chem. Lett. 30 (2019) 2186-2190. DOI:10.1016/j.cclet.2019.08.014 |
| [41] |
Y. Fan, L.C. Wang, Q.J. Xing, et al., Chin. Chem. Lett. 31 (2020) 1648-1653. DOI:10.1016/j.cclet.2019.08.020 |
| [42] |
Y. Peng, M. Cui, Z. Zhang, et al., ACS Catal. 9 (2019) 10803-10811. DOI:10.1021/acscatal.9b02282 |
| [43] |
K. Wang, S. Shu, M. Chen, et al., Chem. Eng. J. 381 (2020) 122673. DOI:10.1016/j.cej.2019.122673 |
| [44] |
W. Fu, S. Shu, J. Li, et al., Nanoscale 11 (2019) 15892-15899. DOI:10.1039/C9NR04634H |
| [45] |
H. Yu, R. Shi, Y. Zhao, et al., Adv. Mater. 29 (2017) 1605148. DOI:10.1002/adma.201605148 |
| [46] |
S. Shu, W.Y. Fu, P. Wang, et al., Appl. Catal. A-Gen. 583 (2019) 117146. DOI:10.1016/j.apcata.2019.117146 |
| [47] |
J. Li, X.A. Dong, Y. Sun, et al., Appl. Catal. B-Environ. 239 (2018) 187-195. DOI:10.1016/j.apcatb.2018.08.019 |
| [48] |
K.L. Li, W. Cui, J.Y. Li, et al., Chem. Eng. J. 378 (2019) 122184. DOI:10.1016/j.cej.2019.122184 |
| [49] |
J.Y. Li, P. Yan, K.L. Li, et al., J. Mater. Chem. A 7 (2019) 17014-17021. DOI:10.1039/C9TA05112K |
| [50] |
P.R. Chen, A. Khetan, F.K. Yang, et al., ACS Catal. 7 (2017) 1197-1206. DOI:10.1021/acscatal.6b02963 |
| [51] |
W. Lai, M.K. Lau, V. Chong, et al., Organomet. Chem. 634 (2001) 61-68. DOI:10.1016/S0022-328X(01)01097-X |
| [52] |
J. Ge, L. Zhang, J. Xu, et al., Chin. Chem. Lett. 31 (2020) 792-796. DOI:10.1016/j.cclet.2019.05.030 |
 2020, Vol. 31
2020, Vol. 31 

