b Key Laboratory of Water and Wastewater Treatment(HUST), Ministry of Housing and Urban-Rural Development(MOHURD), Wuhan 430074, China;
c Nanyang Environment & Water Research Institute(NEWRI), Nanyang Technological University, Singapore 637141, Singapore
As one of the typical pharmaceuticals and personal care products (PPCPs), sulfamethoxazole (SMX) has been widely used in human activities and frequently detected in effluents from municipal wastewater plants, the residual SMX in waters could cause potential negative effects on human health and the ecoenvironment [1]. Over the past decades, advanced oxidation processes (AOPs) based on the generation of highly reactive sulfate radical (SO4·-) and hydroxyl radical (·OH) have been widely reported for effectively destructing recalcitrant PPCPs [2, 3]. As compared to ·OH, SO4·- presents advantages including higher oxidation potential (2.5–3.1 V) in a wide pH range and longer halflife (30-40 μs) [4]. Thus, SO4·- can better contact with target organics before self-quenching during the diffusion process [5]. It also reacts more selectively with the organic compounds containing unsaturated bonds or aromatic π electrons [6].
Usually, Different approaches have been used to activate persulfate (PS), such as increasing temperature, addition of transition metal or chelation agents, or by ultraviolet irradiation (UV) [7]. Among these, iron-based metal activation method was most studied and reported for efficient SO4·- production. And it has also been reported that heterogeneous catalysis with iron oxides are increasingly attractive in replacing homogeneous catalysis of persulfate to prevent the accumulation of soluble iron [8, 9]. However, iron oxides present weak catalytic ability toward PS due to the slow solid-liquid interfacial mass transfer and low generation/regeneration of dissolved Fe2+ [10]. As a common semiconductor, the generation of photo-induced electron on the surface of iron oxides under the irradiation of UV light can also promote the PDS activation as well as the iron reduction. While the wide band width (Eg) and fast electron-hole recombination rate limit their practical use, further modification would be needed [11]. Recently, carbon-based materials have been widely used to enhance the visible light absorbance and electron transfer.
Carbon quantum dots (CQDs) are of exclusive interest as an innovative family of materials for semiconductor photocatalysis for its low cost, upconversion ability and non-toxicity with reliable stability, as well as excellent electron-reservoir and electrontransfer properties [12]. It has been reported that CQDs could improve the photocatalytic activity of iron oxides by separating photogenerated charge carriers by forming heterojunction [13, 14]. Recently, Zhang et al. investigated effective photocatalytic activity of Fe2O3/CQDs nanocomposites for the photodegradation of toxic gas (gas-phase benzene and methanol) under visible light and Zeinab Rabiei et al. investigated Ti/CQD@α-Fe2O3 photoanode for water splitting under visible light irradiation [15, 16]. However, as far as we know, the photoactivation properties of PDS by CQDs modified iron oxide has not been studied yet.
In this study, a CQDs modified maghemite (γ-Fe2O3) catalyst was successfully synthesized by a one-step solvothermal method, which could efficiently activate PDS for rapid SMX degradation under visible light irradiation. The main objectives were to reveal the critical role of CQDs in improving the photochemical ability of the CQDs@γ-Fe2O3 catalyst and explore the reaction mechanism based on the enhanced solid-liquid interfacial iron cycle.
CQDs@γ-Fe2O3 was prepared by a one-step solvothermal synthesis method. Briefly, 0.8 g glucose (as precursor of carbon quantum dots) was dissolved in a 50 mL solution of ethanol:H2O (1.5:1), then 5 g commercial γ-Fe2O3 (Aladdin Company) was dispersed in the mixed solution for 12 h, the above suspension was transferred to a 50 mL Teflon-lined stainless steel autoclave. The autoclave was heated at 180 ℃ for 24 h and then cooled to ambient temperature. The resulting precipitation was collected, washed, and vacuum dried at 60 ℃.
Morphology of the CQDs@γ-Fe2O3 was characterized by a field emission-scanning electron microscopy (FE-SEM, EM3900 M, ZEISS, Germany) equipped with EDAX energy dispersive X-ray spectrometer (EDS) and a high-resolution transmission electron microscopy (HR-TEM, Tecnai G2 F30 transmission electron microscope). X-ray diffraction (XRD) was performed on a Seifert Iso-Debyeflex 2002 diffractometer using Cu–Kα radiation (wavelength =1.54 Å). The Brunauer-Emmett-Teller (BET) surface areas were obtained using a Gemini Ⅶ 2390 analyzer (Micromeritics Instrument Corp. U. S. A.). Zeta potentials were measured by a Zetasizer Nano-ZS90. The involved radicals were characterized by an electron paramagnetic resonance spectrometer (EPR, MEXnano, Bruker), the modulation frequency was 100 kHz and the microwave power were 15 mW.
The photochemical degradation experiments of SMX were performed in a 500 mL jacket-glass reactor at 25 ℃ under the irradiation of a 300 W xenon lamp with light filter, which emitted visible light at 455 nm. In a typical experiment run, the reaction was started as adding 0.2 g/L of CQDs@γ-Fe2O3 into 500 mL reaction solution contain 10 mg/L SMX and 2 mmol/L PDS, the reaction pH was set to 3. Scavenging experiments were carried out in the presence of 500 mmol/L scavengers, i.e. tert-butyl alcohol (TBA), methanol (MeOH) and 10 mmol/L potassium bichromate (K2Cr2O7), respectively.
Figs. 1a–c exhibited the SEM and TEM images of the CQDs@γ-Fe2O3 catalyst. It was found that the catalyst particles were uniform and agglomerative with a quasi-spheral morphology of 10-20 nm size, with evenly surface distribution of Fe, O and C. The lattice spacing of 0.220, 0.295 and 0.480 nm, corresponding to the (100) lattice of CQDs graphitic structure [17] and the (113) and (220) lattices of γ-Fe2O3 respectively [18], indicating heterojunction structure was formed between CQDs and γ-Fe2O3. It has been reported that the heterojunction structure could be benefit for the separation of photo-induced electron hole pair [19]. UV–vis Diffuse Reflectance Spectroscopy (UV–vis DRS) measurements revealed that the Eg of the CQDs/γ-Fe2O3 and γ-Fe2O3 were 2.40 and 2.51 eV, respectively, as shown in Fig. 1e. It indicated that CQDs could effectively improve the electron-hole separation efficiency under visible light irradiation. Fig. 1d showed the XRD patterns of γ-Fe2O3 and CQDs@γ-Fe2O3 nanoparticles. The diffraction peaks centered at 2θ = 31.2°, 33.9°, 44.1°, 52.5°, 57.6° and 63.9° were (220), (311), (400), (422), (511), and (440) planes of γ-Fe2O3 and CQDs@γ-Fe2O3, respectively. The strong peak at 18.1° corresponds to (100) lattice of CQDs graphitic structure in TEM, demonstrating that the fabricated catalyst was CQDs@γ-Fe2O3. The specific BET surface area of CQDs@γ-Fe2O3 increased dramatically after CQDs modification, reaching 101.2 m2/g, four times higher than that of pristine γ-Fe2O3 (24.8 m2/g). In Fig. 1f, the zeta potentials of γ-Fe2O3 and CQDs@γ-Fe2O3 were also examined. The zeta potentials were 14.63 mV and -1.38 mV at pH 3 for γ-Fe2O3 and CQDs@γ-Fe2O3, respectively, indicating the different surface charge distribution properties of two catalysts. The negative charged surface of CQDs@γ-Fe2O3 at pH 3 means it could absorb more dissolved iron species, which was beneficial to the subsequence iron reduction and PDS activation. The transient photocurrent measurements were presented in Fig. S1 (Supporting information). It can be seen that the CQDs@γ-Fe2O3/PDS system possessed higher photocurrent density than the CQDs@γ-Fe2O3 system, indicating more photo-produced electrons were generated and transferred to PDS in the CQDs@γ-Fe2O3/PDS system.

|
Download:
|
| Fig. 1. (a) SEM-EDS, (b and c) HR-TEM and selected area electron diffraction images of CQDs@γ-Fe2O3, (d) XRD patterns, (e) UV–vis DRS spectra and (f) zeta potential measurements of γ-Fe2O3 and CQDs@γ-Fe2O3. | |
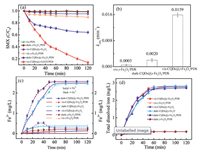
Fig. 2a illustrated the time-dependent degradation of SMX in six comparative systems, i.e. vis-PDS, vis-CQDs@γ-Fe2O3, vis-γ-Fe2O3/PDS, vis-CQDs@γ-Fe2O3/PDS, dark-γ-Fe2O3/PDS and dark-CQDs@γ-Fe2O3/PDS systems. It was found that marginal SMX could be degraded in the vis-PDS, dark-γ-Fe2O3/PDS, indicating that PDS could not be activated by sole visible light or γ-Fe2O3. The poor SMX degradation in the vis-γ-Fe2O3/PDS system also demonstrated the weak photoresponse ability of γ-Fe2O3, perhaps due to the fast electron-hole recombination rate of γ-Fe2O3. Besides, Fig. 2b showed the vis-CQDs@γ-Fe2O3/PDS system could achieve much more rapid SMX degradation rate with a pseudo first-order kinetic constant (kobs) of 1.6 × 10-2 min-1, which was 8.0 and 31.8 times larger than of the dark-CQDs@γ-Fe2O3/PDS and vis-γ-Fe2O3/PDS systems, demonstrating that the loading of CQDs could greatly enhance the photocatalytic activity of the catalyst. As can be seen in the vis-CQDs@γ-Fe2O3 system, there was no SMX could be degraded in the absence of PDS, it proved that SMX could be degraded only when CQDs@γ-Fe2O3 activated PDS during visible light irradiation.
|
Download:
|
| Fig. 2. (a) Comparative SMX degradation and (c) the corresponding simultaneous evolution of dissolved iron species in the vis-PDS, dark-γ-Fe2O3/PDS, vis-CQDs@γ-Fe2O3, vis-γ-Fe2O3/PDS, dark-CQDs@γ-Fe2O3/PDS and vis-CQDs@γ-Fe2O3/PDS systems. (b) The kobs of SMX degradation and (d) release of total dissolved iron in the corresponding reaction systems. Initial conditions were: 0.2 g /L catalyst, 2 mmol/L PDS, 10 mg/L SMX, pH 3 and 25 ℃. | |
Figs. 2c and d shows the time-evolution of dissolved iron species in the γ-Fe2O3/PDS, CQDs@γ-Fe2O3 and CQDs@γ-Fe2O3/PDS systems with visible light irradiation or not. In the vis-γ-Fe2O3/PDS and dark-γ-Fe2O3/PDS systems, the time-dependent releases of iron species were both slight and Fe3+ would be the predominant iron species. It indicated that commercial γ-Fe2O3 would be hardly to photocatalytic activate PDS due to low generation/regeneration of Fe2+ [15]. Interestingly, the assynthesized CQDs@γ-Fe2O3 could lead to relatively remarkable generation of dissolved Fe2+ up to about 2.5 mg/L at 120 min under visible light irradiation, while it leached about 2.4 mg/L Fe3+ and 0.3 mg/L Fe2+ in the corresponding dark case. Moreover, the concentration of Fe3+ would increase from 0 to 0.5 mg/L at the initial 40 min, but obviously decrease to 0.25 mg/L afterwards. This observation suggested that CQDs@γ-Fe2O3 would be visible light sensitive for effectively reductive generation of dissolved Fe2+ catalyst [19].
Apparently, similar dissolved amount of Fe2+/Fe3+ were observed in the CQDs@γ-Fe2O3/PDS systems with visible light irradiation or not, while much more SMX was degraded in the vis-CQDs@γ-Fe2O3/PDS system (Fig. 2a). It indicated that more efficient iron cycles occurred under the co-effect of photo-induced electron reduction and PDS oxidation, leading to the generation of more reactive oxygen species (ROS) in the visible light irradiated system. Furthermore, it was interesting to note that evolution of total released dissolved iron in the four cases using CQDs@γ-Fe2O3 were almost similar with relatively low final amounts of about 2.6–2.7 mg/L. This result suggested that the introduction of CQDs would effectively improve the visible-activation performance of iron oxides materials and lead to acceleration in the solid-liquid interfacial iron cycle for efficient regeneration of Fe2+ [20]. In conclusion, the results of the iron evolution demonstrated that CQDs could greatly improve the photo-responsive behavior of γ-Fe2O3, leading to the faster separation rate of electron and hole. In this case, the photo-induced electrons could transfer to the surface of catalyst and contribute to the in-situ reduction of surface-bounded Fe3+ (Fig. 1f), thus, the more efficient iron cycle in the CQDs@γ-Fe2O3/PDS system would lead to the faster degradation of SMX.
To further confirm the ROS involved in SMX degradation in the CQDs@γ-Fe2O3/PDS system, radical quenching and EPR experiments were conducted. As shown in Fig. S2a (Supporting information), the existence of either MeOH or TBA inhibited the degradation of SMX, with a dramatic decrease of kobs from 1.6 × 10-2 min-1 to 4.0 × 10-4 min-1 and 1.4 ×10-3 min-1, respectively. These results suggest that both ·OH and SO4·- were generated and took part in the reaction [21], while ·OH was relatively more dominant than SO4·- in the degradation process of SMX. Moreover, K2Cr2O7, a scavenger for electrons [22], could entirely inhibit the SMX removal (Fig. S2a), indicating the crucial role of the photo-generated electrons for Fe3+ reduction and PDS activation. As depicted in Fig. S2b (Supporting information), the generation of ·OH and SO4·- in the vis-CQDs@γ-Fe2O3/PDS system were further confirmed by EPR study, notably, the extremely weak signal in the vis-CQDs@γ-Fe2O3 system indicated that PDS rather than O2 was the source of ROS.
To further clarify the reaction mechanism in the vis-CQDs@γ-Fe2O3/PDS system, simulated homogeneous-heterogeneous systems were investigated. Fig. 3a presents the SMX degradation pattern in a homogeneous vis-Fe3+(3 mg/L)/PDS system followed by adding 0.2 g/L CQDs@γ-Fe2O3 at 1 h. It can be seen that SMX could not be degraded in the homogeneous vis-Fe3+/PDS, while 65% of SMX was removed after 1 h by adding CQDs@ γ-Fe2O3. Simultaneous evolution of dissolved iron in Fig. 3b revealed that CQDs@γ-Fe2O3 activated by visible light would effectively reduce the homogeneous Fe2+ and lead to rapid activation of PDS by Fe2+.

|
Download:
|
| Fig. 3. (a) Degradation of SMX in a simulated homogeneous vis-Fe3+(3 mg/L)/PDS system followed by adding 0.2 g/L CQDs@γ-Fe2O3 at 1 h. (b) Comparative degradation of SMX in the dark- and vis-CQDs@γ-Fe2O3/PDS systems after 1 h pre-dissolution under dark or visible light irradiation. (c) and (d) showed the evolution of dissolved iron species in the corresponding systems. Initial conditions were: 0.2 g/L CQDs@γ-Fe2O3, 10 mg/L SMX, pH 3 and 25 ℃. | |
Comparative degradation of SMX in the dark- and vis-CQDs@γ-Fe2O3/PDS systems was further investigated as shown in Fig. 3c, with 1 h pre-dissolution of 0.2 g/L CQDs@γ-Fe2O3/PDS under dark or visible light irradiation, respectively. Once PDS added, it was found that SMX could be rapidly degraded in the vis-CQDs@γ-Fe2O3/PDS system but slowly decomposed in the dark-system. The corresponding evolution of dissolved iron in Fig. 3d indicated that mainly release of Fe2+ from the dark dissolution of CQDs@γ-Fe2O3 would be responsible for the slow SMX degradation. However, visible prfeee-dissolution of CQDs@γ-Fe2O3 would lead to fast dissolved Fe2+, causing the efficient activation of PDS for SMX degradation. In addition, the dissolved iron patterns presented similar evolution with almost Fe2+ in the vis- and dark- systems. Nevertheless, it was interesting to note that SMX could still be continuously degraded in the vis-CQDs@γ-Fe2O3/PDS system. This observation suggested that efficient interfacial regeneration of Fe2+ would occur based on the visible light irradiated CQDs@γ-Fe2O3. As the amount of PDS was enough, the regenerated Fe2+ would be fast consumed to produce SO4·-.
The reusability of the catalysts is crucial in the practical application. To evaluate the catalytic stability of CQDs@γ-Fe2O3 catalyst, the particles were recovered to perform successive tests for SMX degradation (Fig. S3 in Supporting information). It can be seen that the recycled CQDs@γ-Fe2O3 catalyst showed strong activity in SMX degradation and its activity remained almost unchanged in six cycles.
The reaction mechanism in the vis-CQDs@γ-Fe2O3/PDS system was therefore proposed as presented in Fig. 4. The visible irradiation of CQDs@γ-Fe2O3 would be crucial for all the react included in the system. Advantages of the heterojunction structures of CQDs@γ-Fe2O3 would lead to the effective separation of photo-generated electrons and holes. Electrons would be enriched on the surface of CQDs, causing interfacial generation of surface-bond Fe2+ to activate PDS for SMX degradation. An efficient heterogeneous-homogeneous iron cycle would then occur in the vis-CQDs@γ-Fe2O3/PDS system. Fe2+ could be adsorbed on the CQDs surface and rapidly reduced to Fe2+. Meanwhile, the regenerated Fe2+ would lead to the effective Fenton-like reaction of Fe2+/PDS for continuous degradation of SMX.

|
Download:
|
| Fig. 4. Reaction mechanism in the vis-CQDs@γ-Fe2O3/PDS system. | |
The result of this study is expected to provide a novel and easily prepared photocatalyst for visible light activation of PDS. The advantage of CQDs@γ-Fe2O3 was revealed based on an efficient interfacial regeneration of Fe2+ by the photo-generated electrons. However, the electron transfer efficiency upon the specific iron oxides crystal lattices as well as the role of the separated holes are still needed to be further investigation.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis study is financed by the National Natural Science Foundation of China (Nos. 21677055, 21407052), and the Fundamental Research Funds for the Central Universities, Huazhong University of Science and Technology (HUST) (Nos. 2017KFXKJC004, 2016YXMS287). Huazhong University of Science & Technology Analytic and Testing Centre is thanked for the advanced analytic operations.
Appendix A. Supplementary dataSupplementarymaterial relatedtothisarticle canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.01.032.
| [1] |
J. Wang, S. Wang, J. Environ. Manage. 182 (2016) 620-640. DOI:10.1016/j.jenvman.2016.07.049 |
| [2] |
M. Huang, T. Zhou, X. Wu, J. Mao, Water Res. 119 (2017) 47-56. DOI:10.1016/j.watres.2017.03.008 |
| [3] |
H. Zhang, Q. Ji, L. Lai, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 1129-1132. DOI:10.1016/j.cclet.2019.01.025 |
| [4] |
J. Li, Y. Wan, Y. Li, G. Yao, B. Lai, Appl. Catal. B:Environ. 256 (2019) 117782. DOI:10.1016/j.apcatb.2019.117782 |
| [5] |
F. Ghanbari, M. Moradi, Chem. Eng. J. 310 (2017) 42-62. |
| [6] |
Q. Zhao, Q. Mao, Y. Zhou, et al., Chemosphere 189 (2017) 224-238. DOI:10.1016/j.chemosphere.2017.09.042 |
| [7] |
A. Ghauch, A. Tuqan, N. Kibbi, Chem. Eng. J. 279 (2015) 861-873. DOI:10.1016/j.cej.2015.05.067 |
| [8] |
Y. Lei, C. Chen, Y. Tu, Y. Huang, H. Zhang, Environ. Sci. Technol. 49 (2015) 6838-6845. DOI:10.1021/acs.est.5b00623 |
| [9] |
C. Wang, Y. Liu, T. Zhou, et al., Chin. Chem. Lett. 30 (2019) 2231-2235. DOI:10.1016/j.cclet.2019.08.055 |
| [10] |
L. Wei, L. Zhu, W. Nan, et al., Environ. Sci. Technol. 44 (2010) 17-86. DOI:10.1021/es903390g |
| [11] |
T. Zhou, X. Zou, X. Wu, J. Mao, J. Wang, Ultrason. Sonochem. 37 (2017) 320-327. DOI:10.1016/j.ultsonch.2017.01.015 |
| [12] |
Y. Guo, J. Zhang, D. Zhou, S. Dong, J. Mol. Liq. 262 (2018) 194-203. DOI:10.1016/j.molliq.2018.04.091 |
| [13] |
H. Zhou, Q. Sun, X. Wang, et al., Sep. Purif. Technol. 132 (2014) 346-353. DOI:10.1016/j.seppur.2014.05.037 |
| [14] |
F. Ghanbari, M. Moradi, Chem. Eng. J. 310 (2017) 41-62. DOI:10.1016/j.cej.2016.10.064 |
| [15] |
L. Qiu, J. Ma, Sep. Purif. Technol. 210 (2018) 335-342. |
| [16] |
B. Yu, S. Kwak, J. Mater. Chem. 22 (2012) 8345-8353. DOI:10.1039/c2jm16931b |
| [17] |
S. Chandra, A. Chowdhuri, R. Angshuman, et al., J. Nanosci. Nanotechnol. 17 (2017) 1116-1124. DOI:10.1166/jnn.2017.12580 |
| [18] |
X. Chen, X. Duan, W. Ou, et al., Appl. Catal. B 253 (2019) 419-432. DOI:10.1016/j.apcatb.2019.04.018 |
| [19] |
M. Huang, W. Xiang, T. Zhou, et al., Sci. Total Environ. 697 (2019) 134220. DOI:10.1016/j.scitotenv.2019.134220 |
| [20] |
X. Duan, S. Indrawirawan, J. Kang, et al., Sustain. Mater. Technol. 7 (2019) 23904-23913. |
| [21] |
K. Chung, C. Mei, J. Davies, K. Wilson, M. Chan, Atmos. Chem. Phys. 18 (2018) 2809-2820. DOI:10.5194/acp-18-2809-2018 |
| [22] |
G. Dong, L. Yang, F. Wang, ACS Catal. 6 (2016) 6511-6519. DOI:10.1021/acscatal.6b01657 |
 2020, Vol. 31
2020, Vol. 31 

