b Academy of Economics and Environmental Sciences, Xichang University, Xichang 615000, China
Because of uniform color, high tolerance to oxidation and heating conditions, tartrazine (TTZ) is widely used in the food additive and colorant [1, 2]. However, with heavy use in industry and life, the danger of the TTZ gradually emerges. Previous studies had shown that TTZ possibly gave rise to adverse physique influences such as chronic poisoning, hindering children's intellectual development, asthma and carcinogenicity [3]. But as a result of its stability, existing sewage treatment methods cannot completely remove it, and TTZ could accumulate in the environment and might cause greater potential threats [4]. At present, it is imperative to put forward a fast and effective method for TTZ removal. Hasna et al. used zinc-aluminum layered double hydroxide to adsorb TTZ dye from aqueous solution and the removal efficiency reached 98.9% within 60 min. Also, electroFenton reactor was utilized to TTZ degradation by Zhang et al. with the TTZ removal of 80% after 12 h continuous operations. Marija et al. synthesized Co(Ⅱ) impregnated Al(Ⅲ)-pillared montmorillonite to activate peroxymonosulfate (PMS) to degrade TTZ and the decolorization was 93.5% within 120 min [5-7]. These studies have great significance to disposal of TTZ, but the material repeatability, TTZ degradation speed and efficiency limited their application. This study is to find a better method to degrade TTZ.
Owing to the production of reactive oxygen species (ROS, such as hydroxyl radical HO· and sulfate radical SO4·-), advanced oxidation processes (AOPs) can oxidize or degrade refractory macromolecules to reduce their harm [8]. HO· and SO4·- possess higher redox potential (2.8 V and 2.5–3.1 V, respectively), which can contribute to destroy the structure of macromolecules [9-12]. The AOPs have the advantages of rapid reaction, easy application and high activity, so they were gradually taken seriously by people. Ordinarily, people activate PMS by ultrasound, ultraviolet, transition elements, metallic oxide, and thermal energy to produce ROS [13]. However, for economy and convenient operation, the transition elements and metallic oxide look like an optimal PMS activation mode.
Through the above discussion, to propose a catalyst which can activate PMS to generate the ROS for efficient contaminants elimination is meaningful and vital for the application of AOPs in actual water disposal. As a heterogeneous catalyst, compared with homogeneous catalyst, metal oxide is easier to recycle and causes less environmental pollution, it attracts more attention. Owing to the rich reserves, inexpensive and environment friendly characteristics, Fe and Mn oxides are considered as preferred activators for PMS. Huang et al. utilized MnO2 with different structures to activate PMS to degrade Bisphenol A, and the best removal efficiency was 94.5% within 10 min [10]. Ramin et al. obtained 88.5% degradation rate of cyfluthrin through PMS activated by Fe2O3/AC mesoporous heterojunction within 80 min [14]. Ma et al. utilized Fe2O3/Mn3O4 to activate PS (persulfate) for rhodamine B (Rh B) decomposition and achieved good success [15]. These researches, to some extent, prove the feasibility of PMS activation by Fe and Mn oxides to remove organic pollutant. In this work, we synthesized a novel magnetic Fe2O3/Mn2O3 composite by a handy method as an activator of PMS. Compared with the above catalysts, this as-prepared catalyst possesses magnetism in ordertofacilitate recycling and its preparation method is simple. Moreover, we take advantage of Fe2O3/Mn2O3@PMS system to degrade refractory organic TTZ under different conditions to detect its efficiency.
In this study, the magnetic Fe2O3/Mn2O3 composites were synthesized through a handy way and the main materials and agentia usedwere exhibited in Supporting information. Specifically, an appropriate mole proportion of MnSO4·H2O and K3[Fe(CN)6] (with Mn/Fe of 1:1, 3:2 and 2:3) were dissolved in three beakers respectively, stirring to dissolve completely. The mixed suspension was aged at room temperature for 24 h without any interruption, after that, centrifuged and the lower layer precipitate was washed with water and ethanol for 3 times respectively. Then the sediment was put into an oven to dry at 60 ℃ for 8 h. Finally, the dried precipitate was calcined at 450 ℃ for 2 h with the heating rate of 2 ℃/min in the air (CAUTION: Be careful not to burn yourself). Cool to room temperature naturally and the required magnetic Fe2O3/Mn2O3 composites were obtained.
After the synthesis, the morphology and elemental analysis of Fe2O3/Mn2O3 composites catalysts were detected by SEM (scanning electron microscope), EDS (energy dispersive spectrometer) and Mapping (Japan Electron Optics Laboratory Co., Ltd. JSM-6701 F). XRD (X-ray diffraction) atlas of the catalysts was achieved on a D/Max-2004 X-ray powder diffractometer (Bruker, Germany) with Cu Kα radiation (λ = 0.15425 nm). Moreover, XPS (X-ray photoelectron spectroscopy) (Kratos AXIS Ultra DLD) spectrum was done with an Al Kα (1486.6 eV) source for getting a better understanding of the role of Mn, Fe, and O species in the Fe2O3/Mn2O3@PMS system.
Next, we conducted a serious of degradation experiments and the experimental design was as follows. Based on the actual situation, 10 mg/L TTZ was selected as the target contaminant concentration in the work. Put 50 mL of TTZ solution into 150 mL conical flasks and put them in a wobbly machine in which set the oscillation frequency and reaction temperature were 180 rpm and 25 ℃, respectively. Duringeach experiment, a set numberof Fe2O3/Mn2O3 and PMS were added in flasks according to the experimental design. Then, at certain time intermission, 3 mL of the solution was taken from the flasks with a disposable syringe and extracted with a 0.22 μm filter membrane. Immediately the absorbance of the extracting solution was measured by T6 UV–vis spectrophotometer (Persee, Beijing) at the utmost absorption wavelengths of the TTZ (427 nm). Moreover, some pivotal factors that could affect TTZ degradation were detected too. We also degrade some common contaminants (such as Rh B, malachite green (MG), amino black (AB), methyl orange (MO) and methylene blue (MB)) to test the practical applicability of the Fe2O3/Mn2O3@PMS system in this work.
XRD patterns of the Fe2O3/Mn2O3 were displayed in Fig. 1a. As clearly shown, they were all perfectly matched with the standard based on their JCPDS cards. At the XRD pattern of Fe2O3/Mn2O3, a sharp peak (2θ = 35.64°) was related to (104) plane of Fe2O3 (JCPDS No. 73-0603) and other seven intense peaks at 2θ = 30.0°, 32.9°, 42.9°, 53.3°, 57.0°, 60.6° and 62.3° could be indexed to (310), (222), (420), (521), (035), (611) and (026) crystal plane of Mn2O3 (JCPDS No. 76-0150) respectively. In addition, comparing fresh and used XRD pattern of Fe2O3/Mn2O3 (Mn/Fe = 3:2), we could see that although the intensity of some peaks decreased slightly after the reaction, their positions remained basicallyunchanged and no new peaks appeared. To a certain extent, this result proved that the Fe2O3/Mn2O3 composites had been successfully fabricated and possessed outstanding stability.

|
Download:
|
| Fig. 1. XRD patterns of Fe2O3/Mn2O3 composite (a), XPS survey spectra of Fe2O3/Mn2O3 composite (b) and corresponding Mn 2p (c) and Fe 2p peak (d) of the composites (before and after cyclic reaction). | |
XPS measurements were conducted to characterize elemental component and oxidation state of Fe2O3/Mn2O3 composite and the corresponding results were exhibited in Fig. 1b. As shown at Fig. 1b, C 1s, O 1s, Mn 2p and Fe 2p peaks appeared in the XPS full-scale spectrum evidently which demonstrated presence of these elements in the as-prepared samples [16, 17]. Both in the fresh and used samples, three peaks were exhibited at Mn 2p high resolution scan (Fig. 1c), and the position of the peaks had no obvious shift. In addition, based on previous researches, the peaks at 644.4 eV and 642.1 eV corresponded to Mn(Ⅳ) and Mn(Ⅲ) [18, 19]. Moreover, it could be clearly seen in Fig. 1c that approximately 5% Mn(Ⅲ) converted to Mn(Ⅳ) after cyclic reaction. Which was ascribed to the mutual transformation of Mn(Ⅲ)/Mn(Ⅳ) during activating PMS. The Fig. 1d exhibited the condition of Fe element in the degradation experiments. As shown, there were two obvious peaks in the fresh sample and after experiments an extra peak emerged. According to the predecessors' literatures, Fe(Ⅲ) would convert to Fe(Ⅱ) in the activation of PMS which coincided with the above phenomena [20]. These results would be of great significance in the subsequent analysis of reaction mechanism.
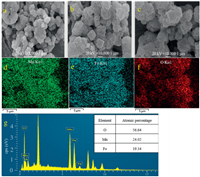
The SEM and Mapping technique were hired to measure the morphology and element distribution of the Fe2O3/Mn2O3 composite (Fig. 2a). The SEM images revealed that this catalyst consists of two substances with different morphologies, cubic structure and agglomerated small particles (Figs. 2a-c), which coincided with previous studies that Mn2O3 present a cube shape, and Fe2O3 showed small granular shape [13-16]. Moreover, Fig. 2c exhibited the SEM image of Fe2O3/Mn2O3 composite which had already participated in five recycles. In contrast to Figs. 2a and b, there was no obvious difference in appearance which implied the composite performs excellently in stability. This was consistent with the XRD described. In addition, the mapping images were applied to display the equidistribution of elements belonging to Fe2O3/Mn2O3 composite (Figs. 2d-f). As shown, Mn, Fe and O atoms were at a coexistence distribution pattern which hinted Fe2O3 and Mn2O3 blending regularly. Finally, the EDS was used to exhibit elemental proportions of the composite (Fig. 2g), and the result was basically in line with the experimental design.
|
Download:
|
| Fig. 2. Fe2O3/Mn2O3 SEM images of before (a, b) and after (c) cyclic reaction, mapping (d, e, f) and EDS (g) of Fe2O3/Mn2O3 composite. | |
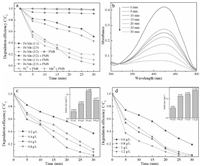
As for the degradation experiments, to evaluate activation performance of Fe2O3/Mn2O3 composite, control experiments were conducted and the outcomes were shown in Fig. 3a. As clearly shown, adsorption effect of three kinds of materials on pollutants could be neglected which indicated their limited adsorption capacity for TTZ. And the degradation rate of TTZ by pure PMS was approximately 8.8% within 30 min which meant it is difficult for separate PMS to exert its catalytic ability effectively. However, after adding the various catalysts, the removal efficiency of TTZ had increased significantly. In detail, the degradation rates raised to 66.7%, 48.8%, 97.3%, 80.2% and 89.7% when added four kinds of catalysts (Fe2O3/Mn2O3 with the mole ratio 1:1, 3:2 and 2:3, homogeneous Fe and Mn ions, respectively). It was clearly observed that with the addition of catalyst Fe2O3/Mn2O3 (mole ratio 2:3), the degradation rate of TTZ had the largest increase. That was owed to the excellent activation capacity of the catalyst for PMS, and then more active species were produced to oxidize TTZ. In addition, Fig. 3b exhibited the strength variation of unique absorbance peaks of TTZ at 427 nm. As seen, with time going on and the reaction proceeding, the peak was gradually decreased until disappeared at 30 min, which indicated TTZ was removed thoroughly by Fe2O3/Mn2O3/PMS system. Based on all of these evidences, the Fe2O3/Mn2O3 catalyst with the mole ratio of 2:3 possessed the better PMS activation performance as a result of the cooperation function of PMS and the catalyst and was used in the next series of experiments.
|
Download:
|
| Fig. 3. Degradation of TTZ in solution under various reaction system (a), UV–vis absorption spectra of TTZ degradation (b), catalyst dosage (c), PMS concentration (d) and the insets were the corresponding initial rate (Reaction conditions: PMS concentration = 0.8 g/L, catalyst dosage = 0.6 g/L, Fe3+ and Mn2+ concentration (FeCl3 and MnCl2) = 0.6 g/L, TTZ concentration = 0.01 g/L, reaction temperature = 25 ℃ and initial pH value = 6.89 (unadjusted)). | |
Besides, it is generally known that the concentration of catalyst is important for the degradation experiments in PMS system, and therefore a serious of tests were conducted to detect the influence of Fe2O3/Mn2O3 dosage with other factors fixed. In addition, the heterogeneous catalytic degradation experiment could be analyzed by the Langmuir-Hinshelwood model (Eq. 1):
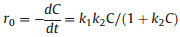
|
(1) |
Then, as the contaminant is removed quickly, the model can be simplified as follow (Eqs. 2 and 3):

|
(2) |
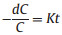
|
(3) |
where r0, k1, k2 and K are the total reaction rate, apparent reaction rate constant, adsorption constant and first order apparent rate constant (min–1), respectively, C and t are the contaminant concentration and reaction time. In this way, we use this simplified model to fit the following degradation experiments and get the corresponding kinetic rate constant. The results were revealed in insertion of the figures. As Fig. 3c illustrated, at low catalyst dosage, the degradation rate of TTZ increased with the growth of the catalyst concentration. In detail, when the catalyst dosage raised from 0.2 g/L to 0.6 g/L, the removal efficiency increased from 62.6%–97.3% and the kinetic rate constant also raised from 0.0305 min-1 to 0.1148 min-1. Noticeably, when the catalyst concentration raised from 0.6 g/L to 0.8 g/L, the degradation rate and kinetic rate constant were down to 92.2% and 0.0777 min-1. This phenomenon could be explicated that when the catalyst dosage was increased, the active sites increased correspondingly. And to a certain extent, more active sites would generate more active species to participate in degradation process. However, when Fe2O3/Mn2O3 was excess, the reaction system had no additional PMS to active and the removal efficiency could not raise evidently. Moreover, the excess catalyst could inhibit the active species and decrease the degradation rate [21]. To sum up, in the Fe2O3/Mn2O3/PMS system, 0.6 g/L was used in the next series of experiments.
Moreover, the influence of PMS dosage on the decomposition experiments was exhibited in Fig. 3d. As shown, the degradation rate of TTZ was direct proportion to the dosage of PMS. Specifically, when the PMS concentration raised from 0.6 g/L to 1.2 g/L, the removal efficiency and kinetic rate constant growth of TTZ were from 80.7% and 0.052 min-1 to 98.7% and 0.1441 min-1, respectively. They have both been promoted evidently. That might be because PMS is the origin of the active species, so more PMS will produce more active species and the degradation rate will grow correspondingly [22]. However, when the PMS dosage added to 1.2 g/L from 1.0 g/L, the removal efficiency and kinetic rate constant of TTZ only had a tiny change. This appearance might attribute to the fact that excessive PMS could boost the side reaction (Eqs. 4–6), and active species would reduce by the ego depletion [21-25].
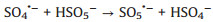
|
(4) |
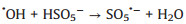
|
(5) |
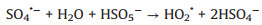
|
(6) |
As a matter of fact, the pH value of the practical water is various in the different places. Therefore, it is of great significance to detect the influence of initial water pH in the degradation experiments. As exhibited in Fig. 4a, in the pH range of 3–9, the trend of removal efficiency of TTZ did not seem to change much, and the degradation rate all achieved nearly 98% within 30 min. Noticeably, when the pH raised to 11, the reaction rate speeded up remarkably. And the degradation rate attained 80% within 5 min, 93.5% within 20 min and finally reached 98% at 30 min. That demonstrated the Fe2O3/Mn2O3/PMS system had a good degradation effect in a wide range of pH. This is quite valuable in practical application. The above result could be interpreted that at the condition of higher concentration of OH-, the inhibition reaction of H+ to active species was weakened and then promoted the speed of TTZ decomposition (Eqs. 7 and 8) [26-28]. In addition, alkaline conditions could accelerate the following reactions of Eqs. 13, 15, 16 and 20 which also led to the raise of TTZ degradation rate.
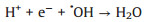
|
(7) |
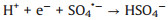
|
(8) |

|
Download:
|
| Fig. 4. Effect of different initial reaction pH (a), extra material addition (b), inhibition experiments (c) and various contaminants degradation (d) in the system of Fe2O3/Mn2O3 composite activated PMS, the inset was the corresponding initial rate (Reaction conditions: PMS concentration = 0.8 g/L, catalyst dosage = 0.6 g/L, TTZ concentration = 0.01 g/L, inhibitor dosage = 50 mmol/L, various contaminants concentration = 0.01 g/L, reaction temperature = 25 ℃ and initial pH 6.89 (unadjusted)). | |
In addition, in order to make the degradation experiment more suitable to the reality, some natural water substances which were abundant in natural waters were appended to the simulated sewage, such as Cl-, HPO42-, HCO3-, NO3- and NOM (nature organic matter). As detailedly exhibited in Fig. 4b, with the addition of HPO42-, HCO3-, NO3- and NOM, the TTZ removal efficiency and kinetic rate constant were reduced in different degree. Moreover, the inhibitory effects were HPO42- (50%, 0.0202 min-1) > HCO3- (83%, 0.0509 min-1) > NO3- (88%, 0.064 min-1) > NOM (92%, 0.0842 min-1) after 30 min reaction. However, the degradation experiment was accelerated after the adding of Cl- and the degradation rate reached 100% within 30 min. The reasons of this phenomenon were listed below: (ⅰ) HPO42- performed a strong negative effect on TTZ decomposition, which imputed the reaction of HPO42- and active species (Eqs. 9 and 10). Both ·OH and SO4·- could be eliminated and the degradation rate naturally decreased [27, 29, 30]. (ⅱ) HCO3- also could react with ·OH and SO4·- and generated some free radicals with low redox potential, such as CO3·- (E0 = 1.78 V) and HCO3· (E0 = 1.65 V) (Eq. 11). Moreover, HCO3- could react with PMS directly as well and produced peroxymonocarbonate (HCO4-, E0 = 1.8 V). Then HCO4- was unstable and would decomposed into hydrogen peroxide and HCO3- (Eqs. 12 and 13). These factors resulted in the reduction of TTZ decomposition [29, 31, 32]. (ⅲ) According to the previous researches, NO3· could generate by the reactions of NO3- with active species. And NO3· possessed low oxidability, thus mitigated the progress of degradation [33]. The specific reactions were shown in Eq.14. (ⅳ) In this research, humic acid (HA) was used to detect the influence of NOM on the TTZ degradation. HA could unite with the Fe2O3/Mn2O3, and obstruct the PMS activation process. In this way, it exhibited some inhibition effect in the Fe2O3/Mn2O3/PMS system [34]. Furthermore, after the addition of Cl-, PMS reacted with it and produced HClO which could promote TTZ degradation (Eq. 15) [35-37].
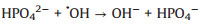
|
(9) |
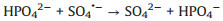
|
(10) |
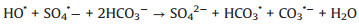
|
(11) |
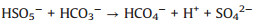
|
(12) |

|
(13) |
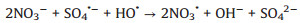
|
(14) |
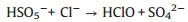
|
(15) |
And then, radical inhibition experiments were conducted to detect the function of HO· and SO4·- at the decomposition of TTZ. Generallyspeaking, ethanol(EtOH)(SO4·-(1.6–7.7)×107Lmol-1s-1 and HO·(1.2–2.8)×109 L mol-1 s-1) and tertiary butanol (TBA) (HO· (3.8–7.6) × 108 L mol-1 s-1) acted as common inhibitors in the experiment [35-40]. Consequently, they were employed to radical clearance test in this work, and the results were displayed in Fig. 4c. As illustrated, with no inhibitors the degradation rate reached 97.3%, however after the mingling of TBA and EtOH it decreased to 43.7% and 29.4% within 30 min respectively. Both TBA and EtOH inhibited the TTZ degradation, in otherwords, both HO· and SO4·- playeda key role in the experiment. Moreover, in order to explore the practicability of Fe2O3/Mn2O3/PMS system, a serious of degradation experiments for various organic pollutants were carried out, such as Rh B, MG, AB, MO and MB. Fig. 4d exhibited the degradation results, the removal efficiency were 100%, 92.7%, 81.6%, 95.5% and 100% respectively, which indicated these organic pollutants could be effectively degradedwithin30 minattheFe2O3/Mn2O3/PMSsystem. From the above analyses, the following conclusions could be drawn: In the Fe2O3/Mn2O3/PMS system, both HO· and SO4·- were of great significance for TTZ degradation. In addition, by degrading various organic pollutants, the practicability of this system was affirmed.
Finally, for the catalyst reusability, cycle experiments were conducted for 5 times, and the outcomes were exhibited in Fig. S1a (Supporting information). Moreover, owning to the incorporation of magnetic Fe2O3, this catalyst was provided with certain magnetic properties. Fig. S1b (Supporting information) exhibited the magnetism of catalyst, and Fe2O3/Mn2O3 were drawn to an external magnet without difficulty within 1 min, which were significant to the recycling. As seen, the degradation rate of TTZ within 30 min stabled at about 95% basically in this five cyclic experiments. Meanwhile, the ions leaching of Fe2O3/Mn2O3 were also measured and the ion concentration released from Fe2O3 and Mn2O3 were 0.136 mg/L and 0.006 mg/L which were 0.02% of the total catalyst. Therefore, based on these results and the XRD atlases, the Fe2O3/Mn2O3 catalyst possessed excellent stability and was more environmentally friendly and it is a promising catalyst.
According to the debate above and previous researches, the potential mechanism of the Fe2O3/Mn2O3/PMS system for TTZ degradation was proposed. Firstly, pure PMS reacted with TTZ directly without the participation of active species (Eqs. 16). And then, Mn(Ⅲ) and Fe(Ⅲ) on Fe2O3/Mn2O3 surface activated PMS to produce SO4·- and SO5·- respectively (Eqs. 17 and 18). Because of this, part of the Mn(Ⅲ) and Fe(Ⅲ) transformed to Mn(Ⅳ) and Fe(Ⅱ), which was consistent with the XPS atlases result [19]. Meanwhile, the generated Mn(Ⅳ) and Fe(Ⅱ) were able to re-form Mn(Ⅲ) and Fe(Ⅲ) by reacting with HSO5- (Eqs. 19–21). In addition, because the oxidability of Mn(Ⅳ) is stronger than Fe (Ⅲ), the Eq. (22) could take place to regenerate Fe(Ⅲ) and meanwhile Mn(Ⅳ)/Mn(Ⅲ) redox pairs become the main role that responsible for PMS activation. It was precisely for this reason that the TTZ degradation experiments could carry on and active species could generate continuously. In addition, the SO5·- could break down into SO4·- and O2 in aqueous solution (Eq. 23). SO4·- was able to react with H2O as well to generate HO· (Eq. 24) [41-47]. Finally, all of the HO· and SO4·- completely oxidized and decomposed TTZ (Eq. 25) and the thorough oxidation reactions were displayed below:
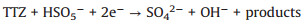
|
(16) |
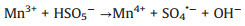
|
(17) |
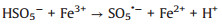
|
(18) |

|
(19) |
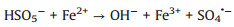
|
(20) |
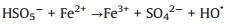
|
(21) |
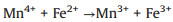
|
(22) |
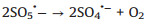
|
(23) |
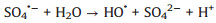
|
(24) |

|
(25) |
In summary, the Fe2O3/Mn2O3 catalyst was synthesized by simple two-step method in this work, and several methods (XRD, SEM, EDS, Mapping and XPS) were conducted to characterize it. And then, TTZ decomposition experiments were carried out under various conditions in the Fe2O3/Mn2O3/PMS system to detect the activation properties of catalysts. The discussion results showed that (ⅰ) through a series of characterization experiments, the Fe2O3/Mn2O3 catalyst was proved to be successfully prepared. (ⅱ) Different degradation experiments confirmed the great PMS activation capacity of Fe2O3/Mn2O3 (Fe/Mn = 2:3), the removal efficiency of TTZ (10 mg/L) reached 97.3% within 30 min under the Fe2O3/Mn2O3/PMS system. The optimum reaction conditions were also found, such as catalyst dosage (0.6 g/L), PMS concentration (0.8 g/L) and the initial pH 11. In addition, inhibitor experiments indicated both the HO· and SO4·- played a vital role in the experiments. And HPO42-, HCO3-, NO3- and NOM could slow down the experiments progressing while Cl- could boost it. (ⅲ) Reusability and ions leaching experiments as well as the used catalyst XRD and SEM images implied the excellent stability and cyclicity of the Fe2O3/Mn2O3 composite. (ⅳ) Based on the XPS and the experiments results, the possible mechanism of TTZ degradation was proposed.
Declaration of competing interestWe have no conflicts of interest to declare.
AcknowledgmentsThis work was kindly funded by the National Natural Science Foundation of China (No. 51978319), National College Student Innovation and Entrepreneurship Training Program of Lanzhou University and Key Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources, Qinghai Institute of Salt Lake, Chinese Academy of Sciences.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.02.033.
| [1] |
Y. Wang, Y. Mu, J. Hu, Q. Zhuang, Y. Ni, Spectrochim. Acta A: Mol. Biomol. Spectrosc. 214 (2019) 445-450. DOI:10.1016/j.saa.2019.02.055 |
| [2] |
S. Sahnoun, M. Boutahala, Int. J. Biol. Macromol. 114 (2018) 1345-1353. DOI:10.1016/j.ijbiomac.2018.02.146 |
| [3] |
S. Balu, S. Velmurugan, S. Palanisamy, S.W. Chen, J. Taiwan Inst, Chem. E 99 (2019) 258-267. |
| [4] |
C. Zhang, G. Ren, W. Wang, et al., Sep. Purif. Technol. 208 (2019) 76-82. DOI:10.1016/j.seppur.2018.05.016 |
| [5] |
H. Ouassif, E.M. Moujahid, R. Lahkale, et al., Surf. Interface Anal. 18 (2020) 100401. DOI:10.1016/j.surfin.2019.100401 |
| [6] |
M. Marković, S. Marinović, T. Mudrinić, et al., Appl. Clay Sci. 182 (2019) 105276. DOI:10.1016/j.clay.2019.105276 |
| [7] |
Z. Wang, X. Zhang, H. Zhang, et al., Sep. Purif. Technol. 215 (2019) 528-539. DOI:10.1016/j.seppur.2019.01.063 |
| [8] |
R. Guo, Q. Meng, H. Zhang, et al., Chem. Eng. J. 355 (2019) 952-962. DOI:10.1016/j.cej.2018.08.168 |
| [9] |
Q. Ma, H. Zhang, X. Zhang, et al., Chem. Eng. J. 360 (2019) 848-860. DOI:10.1016/j.cej.2018.12.036 |
| [10] |
H. Zhang, J. Wang, X. Zhang, B. Li, X. Cheng, Chem. Eng. J. 369 (2019) 834-844. DOI:10.1016/j.cej.2019.03.132 |
| [11] |
N. Tian, X. Tian, Y. Nie, et al., Chem. Eng. J. 355 (2019) 448-456. DOI:10.1016/j.cej.2018.08.183 |
| [12] |
J. Huang, Y. Dai, K. Singewald, et al., Chem. Eng. J. 370 (2019) 906-915. DOI:10.1016/j.cej.2019.03.238 |
| [13] |
R. Khaghani, B. Kakavandi, K. Ghadirinejad, E. Dehghani Fard, A. Asadi, Microporous Mesoporous Mater. 284 (2019) 111-121. DOI:10.1016/j.micromeso.2019.04.013 |
| [14] |
Q. Ma, X. Zhang, R. Guo, et al., Sep. Purif. Technol. 210 (2019) 335-342. DOI:10.1016/j.seppur.2018.06.060 |
| [15] |
J. Zhao, J. Nan, Z. Zhao, N. Li, Catal. Commun. 102 (2017) 5-8. DOI:10.1016/j.catcom.2017.08.018 |
| [16] |
N. Parveen, Z. Khan, S.A. Ansari, et al., Chem. Eng. J. 360 (2019) 415-422. DOI:10.1016/j.cej.2018.11.228 |
| [17] |
M. Nasiri, P. Sangpour, S. Yousefzadeh, M. Bagheri, J. Environ, Chem. Eng. 7 (2019) 102999. |
| [18] |
D. Wang, L. Jin, Y. Li, et al., Fuel Process. Technol. 191 (2019) 20-28. DOI:10.1016/j.fuproc.2019.03.024 |
| [19] |
Q. Yang, X. Yang, Y. Yan, et al., Chem. Eng. J. 348 (2018) 263-270. DOI:10.1016/j.cej.2018.04.206 |
| [20] |
J. Deng, S. Feng, X. Ma, et al., Sep. Purif. Technol. 167 (2016) 181-189. DOI:10.1016/j.seppur.2016.04.035 |
| [21] |
G. Chen, L.C. Nengzi, B. Li, et al., Sci. Total Environ. 695 (2019) 133963. DOI:10.1016/j.scitotenv.2019.133963 |
| [22] |
G. Chen, X. Zhang, Y. Gao, et al., Sep. Purif. Technol. 213 (2019) 456-464. DOI:10.1016/j.seppur.2018.12.049 |
| [23] |
J. Li, Y. Wan, Y. Li, G. Yao, B. Lai, Appl. Catal. B:Environ. 256 (2019) 117782. DOI:10.1016/j.apcatb.2019.117782 |
| [24] |
J. Yan, J. Li, J. Peng, et al., Chem. Eng. J. 359 (2019) 1097-1110. DOI:10.1016/j.cej.2018.11.074 |
| [25] |
Z. Wang, L.C. Nengzi, X. Zhang, Z. Zhao, X. Cheng, Chem. Eng. J. 381 (2020) 122517. DOI:10.1016/j.cej.2019.122517 |
| [26] |
F. Ghanbari, M. Ahmadi, F. Gohari, Sep. Purif. Technol. 228 (2019) 115732. DOI:10.1016/j.seppur.2019.115732 |
| [27] |
F. Ghanbari, C.A. Martínez-Huitle, J. Electroana. Chem. 847 (2019) 113182. DOI:10.1016/j.jelechem.2019.05.064 |
| [28] |
M. Ahmadi, F. Ghanbari, Environ. Sci. Pollut. Res. Int. 25 (2018) 6003-6014. DOI:10.1007/s11356-017-0936-8 |
| [29] |
Y.H. Huang, Y.F. Huang, C.I. Huang, C.Y. Chen, J. Hazard. Mater. 170 (2009) 1110-1118. DOI:10.1016/j.jhazmat.2009.05.091 |
| [30] |
N. Jaafarzadeh, F. Ghanbari, M. Moradi, Korean J. Chem. Eng. 32 (2015) 458-464. DOI:10.1007/s11814-014-0263-4 |
| [31] |
M. Ahmadi, F. Ghanbari, Mater. Res. Bull. 111 (2019) 43-52. DOI:10.1016/j.materresbull.2018.10.027 |
| [32] |
J. Li, M. Xu, G. Yao, B. Lai, Chem. Eng. J. 348 (2018) 1012-1024. DOI:10.1016/j.cej.2018.05.032 |
| [33] |
M. Nie, Y. Deng, S. Nie, et al., Chem. Eng. J. 369 (2019) 35-45. DOI:10.1016/j.cej.2019.03.046 |
| [34] |
X. Xu, D. Tang, J. Cai, et al., Appl. Catal. B:Environ. 251 (2019) 273-282. DOI:10.1016/j.apcatb.2019.03.080 |
| [35] |
C. Li, Y. Huang, X. Dong, et al., Appl. Catal. B:Environ. 247 (2019) 10-23. DOI:10.1016/j.apcatb.2019.01.079 |
| [36] |
P. Duan, T. Ma, Y. Yue, et al., Environ. Sci. Nano 6 (2019) 1799-1811. DOI:10.1039/C9EN00220K |
| [37] |
M. Ahmadi, F. Ghanbari, A. Alvarez, S. Silva Martinez, Korean J. Chem. Eng. 34 (2017) 2154-2161. DOI:10.1007/s11814-017-0122-1 |
| [38] |
S. Wang, J. Wang, Chem. Eng. J. 379 (2020) 122361. DOI:10.1016/j.cej.2019.122361 |
| [39] |
R. Xie, J. Ji, K. Guo, et al., Chem. Eng. J. 356 (2019) 632-640. DOI:10.1016/j.cej.2018.09.025 |
| [40] |
Y. Huang, X. Zhang, G. Zhu, et al., Sep. Purif. Technol. 215 (2019) 490-499. DOI:10.1016/j.seppur.2019.01.024 |
| [41] |
Y. Shang, C. Chen, P. Zhang, et al., Chem. Eng. J. 375 (2019) 122004. DOI:10.1016/j.cej.2019.122004 |
| [42] |
C. Chen, T. Ma, Y. Shang, et al., Appl. Catal. B:Environ. 250 (2019) 382-395. DOI:10.1016/j.apcatb.2019.03.048 |
| [43] |
C. Gong, F. Chen, Q. Yang, et al., Chem. Eng. J. 321 (2017) 222-232. DOI:10.1016/j.cej.2017.03.117 |
| [44] |
H. Lu, M. Sui, B. Yuan, J. Wang, Y. Lv, Chem. Eng. J. 357 (2019) 140-149. DOI:10.1016/j.cej.2018.09.111 |
| [45] |
J. Deng, Y. Ge, C. Tan, et al., Chem. Eng. J. 330 (2017) 1390-1400. DOI:10.1016/j.cej.2017.07.137 |
| [46] |
C. Huang, Y. Wang, et al., Sep. Purif. Technol. 230 (2020) 115877. DOI:10.1016/j.seppur.2019.115877 |
| [47] |
E. Saputra, H. Zhang, Q. Liu, H. Sun, S. Wang, Chemosphere 159 (2016) 351-358. DOI:10.1016/j.chemosphere.2016.06.021 |
 2020, Vol. 31
2020, Vol. 31 

