b State Environmental Protection Key Laboratory of Simulation and Control of Groundwater Pollution, Chinese Research Academy of Environmental Sciences, Beijing 100012, China;
c State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China;
d Hebei Engineering Research Center for Geographic Information Application, Institute of Geographical Sciences, Hebei Academy of Sciences, Shijiazhuang 050030, China;
e Hebei Provincial Academy of Ecological and Environmental Science, Shijiazhuang 050037, China
Hexavalent chromium (Cr(Ⅵ)) is a widespread contamination in the environment because of its widespread application in various industrial processes such as dyes, electroplating, paints, and leather tanning [1, 2]. Chromium could be existed in the several valence states, ranging from Cr(Ⅱ) to Cr(Ⅵ), but hexavalent and trivalent (Cr(Ⅲ)) are the most common and stable forms in the environment [3]. Compared to Cr(Ⅲ), Cr(Ⅵ) is highly toxic and carcinogenic to the living organisms. Meanwhile, it have severe environmental risks because the typical forms (Cr2O72-, HCr2O72-or CrO4-) are highly mobile and failed to be sorb strongly by soil colloid [4]. Therefore, the conventional Cr(Ⅵ)-contaminated remediation techniques were based on reduction of Cr(Ⅵ) to Cr(Ⅲ), which blocked the entry of Cr(Ⅵ) into the ecosystem [5, 6]. Compared with chemical methods (using reducing agent e.g., nZⅥ, ferrous sulphate) [6, 7], the costeffective and environmentally friendly bio-reduction technology grows to be the most sustainable technology for the reduction of Cr(Ⅵ) to Cr(Ⅲ).
Composting is an effective and low-environmental risk option of disposing organic solid waste in which organic matter can be converted into humic substances (HSs) [8]. Currently, the compost production has received increasing attention due to its multiple environmental applications, such as enriching the soil with source of nutrients for the growth and development of plants (e.g., nitrogen, phosphorus, and potassium), improving microbial activity and accelerating nutrient mobilization [9, 10]. In addition, the compost-derived HSs are the redox-active substance, and the redox capacities are similar to those of soil-derived HSs [11, 12]. It is widely known that the Cr(Ⅵ) can be reduced by the soil-derived HSs, which have a significant impact on its speciation and transport in soil and wetland systems [13, 14]. The HSs could provide both the electron donors and adsorption sites for Cr(Ⅵ) and reduced Cr(Ⅲ), resulting in the significant retention effect when the extrinsic Cr(Ⅵ) entered the soil or wetland systems [14]. For example, Ohta et al. (2012) showed that phenolic group was the mainly functional groups in HSs converting Cr(Ⅵ) to Cr(Ⅲ) [15]. Zhang et al. (2017) found that Cr(Ⅵ) was first adsorbed by carboxyl and ester, and was then reduced from Cr(Ⅵ) to Cr(Ⅲ) by phenol and polysaccharide, forming complexes of the generated Cr(Ⅲ) with carboxylic groups [13]. Therefore, the Cr(Ⅵ) reduction is significantly related to the redox-active functional groups [13, 16]. However, few studies have been reported the reactions between the compost-derived HSs and Cr(Ⅵ). The main functional components of compost-derived HAs impacting Cr(Ⅵ) bioreduction remains unclear.
Iron mineral plays the key role in the biogeochemical and environmental Cr(Ⅵ)-reduction reaction [17]. Previous studies had been devoted to understanding Cr(Ⅵ) reduction involving HSsmineral-microbe interactions in the natural environment [17, 18]. The irons on the surface of mineral could be used as active participants by accepting or donating electrons as in the redox transformations between Cr(Ⅵ) and Cr(Ⅲ) [5, 6]. What is more, HSs could be used as the electron shuttle accelerating reduction of Fe(oxy)hydroxide minerals in the process of dissimilatory iron mineral reduction [19, 20], and then the reduced Fe(Ⅱ) was able to convert Cr(Ⅵ) to Cr(Ⅲ). Cr(Ⅵ) reduction by the structural Fe(Ⅱ) was accomplished via a two-step process. Cr(Ⅵ) was firstly sorbed to the positively-changed iron mineral at neutral pH, and then reduced by the structural Fe(Ⅱ) [18]. Therefore, we hypothesized that the redox-active capacities of compost-derived HSs would have a significant effect for Cr(Ⅵ) reduction from microbialmineral interactions. To clarify the effect, Shewanella oneidensis (S. oneidensis) MR-1 was used as the iron-and Cr(Ⅵ)-reducing bacterium in this work. Meanwhile, hematite (Fe2O3), a common Fe(Ⅲ)-oxides in natural soil environments, was used to identify the effect of Fe(Ⅲ)-mineral reduction in process of Cr(Ⅵ) bioreduction.
In this work, humic acids (HAs) from the municipal solid waste (MSW) compost were used as the representative of HSs to evaluate its effects on Cr(Ⅵ) reduction [21]. The specific objectives were (ⅰ) to describe the compost-derived HSs mediated Cr(Ⅵ) bioreduction with or without iron mineral and (ⅱ) to identify the relationship between active-reduction property of compost-derived HSs and Cr(Ⅵ) reduction. The results of this work will provide the meaningful information to understand the effects of compostderived HSs on Cr(Ⅵ) reduction, and paves a useful way for the in situ remediation of Cr-contaminated soil.
The details of materials used in this study were listed in Text S1 (Supporting information) and all of the analytical methods were supplied in Text S2-S5 (Supporting information).
Samples of different composting stages were collected (0, 7 14, 21, 28 and 51 d), and the compost-derived HAs were extracted based on the IHSS method. Electron transfer capacity (ETC) contained electron donating capacity (EDC) and electron accepting capacity (EAC). Based on our previous study [22], the EDC of compost-derived HAs ranged from 972.45 micromoles per gram of carbon (μmol/(g C)) to 1308.33 μmol/(g C) (Fig. S1 in Supporting information), which is similar to the EDC of the terrestrial HAs using the same method [12, 23]. Elliott soil humic acid (ESHA) and Pahokee Peat humic acid (PPHA) were the typical terrestrial HAs. Owing to the comparability of EDC and EAC between the compostderived HAs and the terrestrial HAs, the reduction of Cr(Ⅵ) mediated by the compost-derived HAs and the terrestrial HAs were conducted under the anoxic, neutral (pH 7) and motionless conditions, and the reduction kinetics were shown in the Fig. S2 (Supporting information). The negligence of Cr(Ⅵ) reduction was observed in the terrestrial HA and the late-stage compost-derived HAs (i.e., C28 and C51). The result was similar to the previous studies which found that the Cr(Ⅵ) reduction by terrestrial HAs was extremely slow at pH 7 [24, 25]. Meanwhile, although the rate was very slow, the Cr(Ⅵ) could still be reduced by the initial-stage compost-derived HAs (i.e., C0 and C7). The reaction rate constants (k) of the compost-derived HAs reduced from 0.137 d-1 to 0.034 d-1, indicated that the humification process of compost-derived HAs might suppress the Cr(Ⅵ) reduction. Overall, the result revealed that the low aromaticity and humification of the compost-derived HAs (the initial-stage compost-derived HAs) could induced Cr(Ⅵ) reduction at pH 7.
The interaction of Cr(Ⅵ) and HAs were investigated by UV–vis spectra (Fig. S3 in Supporting information). The UV–vis spectra revealed two absorption bands at λmax 277 and λmax 351 nm, which were assigned to the structure of O-Cr(Ⅵ). As shown in Fig. S3 (Supporting information), the absorption peaks of Cr(Ⅵ) were significantly decreased after it was treated by the initial-stage compost-derived HA (i.e., C0, C7 and C14). The negligent decreases of absorption peaks of Cr(Ⅵ) were observed after using terrestrial HAs and the late-stage compost-derived HAs. The result further suggested that the compost-derived HAs with the low humification could be used as electron donor to reduce Cr(Ⅵ). In addition, there were not the absorption peaks assigned to the typical octahedral Cr(Ⅲ) structure. The phenomenon might be correlated to its relatively low solubility of Cr(Ⅲ) at pH 7 [26].
Previous investigations suggested that the Cr(Ⅵ) reduction caused by HA included direct and indirect reduction mechanisms [27]. Owing to reactions between the HA and Cr(Ⅵ) were conducted in the phosphate buffered solution with high concentration (50 mmol/L), it was easy to accept that the direct reduction mechanism was dominant because the HPO42- and HPO42- with high concentration were considered to be able to desorbed nearly all the ion exchangeable Cr(Ⅵ) on HA [28]. Phenol, polysaccharide and methyl were able to act as major electron donors for the direct Cr(Ⅵ) reduction [16]. To further confirm the mechanism of Cr(Ⅵ) reduction by the functional groups of the compost-derived HA, the three-dimensional excitation-emission matrix (3D-EEM) was employed to reveal the variations of aromatic structures. The change of the fluorescence intensities, originating from the typical peak of the dissolved organic matter, was investigated by electron transfer. For example, reduction of dissolved organic matter by NaBH4 produced substantially-enhanced, blue-shifted emission arising from local donor states [29]. As shown in Fig S4 (Supporting information), the fluorescence intensities of the typical humic-like peak of the HA showed no change during Cr(Ⅵ) reduction, possibly implied that the ET was not generated in the aromatic structures of the compost-derived HAs during the Cr(Ⅵ) reduction. The functional groups, which were involved in Cr(Ⅵ) reduction, were not the aromatic structures of HAs at pH ~7. Thus, the result indicted that the interaction of CrO42- with the compost-derived HAs might be responding for the non-aromatic functional groups in its inherent constructions, e.g., polysaccharide.
The HA-mediated Cr(Ⅵ) bioreduction were investigated under the anoxic and pH 7. S. oneidensis MR-1 was a humic-and Cr(Ⅵ)-reducing bacterium, and the sodium lactate was used as the electron donor. The kinetics of Cr(Ⅵ) bioreduction by S. oneidensis MR-1 were well fitted by the first order reaction model (Fig. S5 in Supporting information). In the treatment of the MR-1 + HA + Cr(Ⅵ) (with HA), the Cr(Ⅵ) bioreductions in presence of the compost-derived HAs and terrestrial HAs were significantly higher than the control experiences (without HA) during 13 days incubation. For instance, the Cr(Ⅵ) reductions with adding the compost-derived HAs increased by 14.1%–40.3% after the 13 days incubation (Table S2 in Supporting information), indicating that the compost-derived HAs all had a positive influence for the Cr(Ⅵ) bioreduction in the anoxic environment. This was consistent with previous studies which HSs were the significant electron shuttles accelerating the reduction of nitrobenzene, Hg(Ⅱ), As(V) and Sb(V) [11, 30-32]. Meanwhile, as shown in Fig. S5 and Table S2 (Supporting information), the increase rates of Cr(Ⅵ) reduction in presence of PPHA and ESHA after the 13 days incubation were 35.2% and 65.0%, respectively. The result showed that the Cr(Ⅵ) bioreduction of compost-derived HAs were relative weaker than the terrestrial HAs.
For better comparison of the individual experiments, the Cr(Ⅵ) bioreduction rates (kMR-1+HA+Cr(Ⅵ)) were re-drawn in the presence of compost-derived HAs and terrestrial HAs (Fig. 1). In comparison, the HAs extracting from the different composting stages had the remarkable difference of effect in the process of Cr(Ⅵ) bioreduction (Fig. 1a). This dissimilarity depended on the amount of the redox-active functional groups (i.e., quinone) in the compostderived HSs [12, 23]. It was worth noting that the reduction rates of the compost-derived HA were reduced from 0.056 to 0.038 (Fig. 1a), indicating that composting process might have a negative effect in the process of the compost-derived HAs mediated Cr(Ⅵ) bioreduction. Based on the previous researches, the redox properties of compost-derived HAs increased during composting, owing to the formed aromatic functional groups (such as phenolic, carboxyl, quinone) in the inherent structure [8, 33]. However, the result revealed that the Cr(Ⅵ) bioreductions were not significantly associated with the redox capacities of compost-derived HAs. The opposite trends might be attributed to the functional groups governing the directly electron transfer from HAs to Cr(Ⅵ) [13, 34]. In particular, organic compounds containing heteroatoms, such a phenol and polysaccharide, has been previously described as Cr(Ⅵ) reduction agents [13, 35, 36]. For instance, Cr(Ⅵ) was reduced through the formation of a Cr(Ⅵ)-phenol ester complex, followed by the transfer of one electron to Cr(Ⅵ) to production Cr(V) [13].

|
Download:
|
| Fig. 1. Cr(Ⅵ) reduction rate (kMR-1+HA+Cr(Ⅵ)) with the 13 days cultivation in the HA-only system. (a) The distribution of k; (b) Ratio of kt/k0; kt/k0 was calculated from the ratios of the Cr(Ⅵ) reduction with HAs divided by the Cr(Ⅵ) reduction without HAs. | |
Hematite (Fe2O3) was selected for further investigating stimulated Cr(Ⅵ) bioreduction. As shown in Fig. 2a, compostderived HAs could significantly promote the Cr(Ⅵ) reduction in the Fe2O3-HA experiments compared with the control experiment. The contributions of compost-derived HAs in the Fe2O3-HA systems were correlated with the composting time (Fig. 2a), which were different from the contribution of compost-derived HAs in the HAonly systems (without Fe2O3). At the end of batch culture at 13 days, compared with the control experiments, Cr(Ⅵ) bioreduction rate of the compost-derived HA ranged from 113.5%–228.5% times increase (Fig. S6 and Table S3 in Supporting information). Meanwhile, compared to the control experiment, the Cr(Ⅵ) bioreduction rate of ESHA and PPHA were also 2.46 and 2.58 fold increase, respectively (Table S3). Compared to the Fe2O3-only systems (the control experience), the reduction rate (indicated by k value) with the compost-derived HAs addition increased by 235–5.09 times, which were higher than that in HA-only systems (1.36–2.00) (Fig. S7 in Supporting information). After Fe2O3 addition, the Cr(Ⅵ) bioreduction rate increased significantly. These results indicated that electron transfer for the Cr(Ⅵ) bioreduction was significantly responsible for Fe(Ⅲ) oxide reduction. According to the Figs. 1b and 2b, the compost-derived HAs could enhance the Cr(Ⅵ) bioreduction, promoting electron transfer between microorganism and Cr(Ⅵ).

|
Download:
|
| Fig. 2. Cr(Ⅵ) reduction rate (kMR1+HA+Fe2O3+Cr(Ⅵ)) with the 13 days cultivation in Fe2O3-HA systems. (a) The distribution of k; (b) ratio of kt/k0. The kt/k0 was calculated from the ratios of the Cr(Ⅵ) reduction with HAs divided by the Cr(Ⅵ) reduction without HAs. | |
In addition to the compost-derived HAs, PPHA and ESHA were also used to evaluate the influence of Cr(Ⅵ) bioreduction in the Fe2O3-HA experiments. The reduction rates of PPHA and ESHA were 0.158 d-1 and 0.138 d-1, respectively, which were higher than that of the compost-derived HA (0.054-0.117 d-1) (Fig. 2a). The results indicated that the influence of compost-derived HAs were slightly weaker than that of the terrestrial HAs during the Cr(Ⅵ) bioreduction process. Overall, the results clearly showed that compost-derived HAs could use functional groups as the electron transfer groups coupled with the reduction of iron mineral to promote bioreduction Cr(Ⅵ), which was similar to the soil-derive HAs [20, 33, 34]. Electrons was generated by oxidation of organic carbon substrate (sodium lactate in this study), and eventually transferred to Cr(Ⅵ) [3]. This transfer of extracellular electrons was able to be greatly enhanced by adding compost-derived HAs or terrestrial HAs based on the above results. These differences between compost-derived HAs and terrestrial HAs are originated from the different redox-active surface functional groups in the inherent structure, thus the correlation analysis is used to reflect the relationships between the reduction rates of Cr(Ⅵ) and their electron transfer capacities [11, 12].
Electron transfer capacities of compost-derived HAs had been characterized in our previous paper [22]. When the ratios of kt/k0 were plotted vs. the EAC (Fig. 3a) and EDC (Fig. 3b) in the Fe2O3-HA experiments, kt/k0 positively was correlated well to EAC with the high correlation coefficient (R) of 0.905. The correlation coefficient (R) between kt/k0 and EDC was 0.774, indicating that kt/k0 is positive correlated to EDC but unremarkable. EAC/ETC or EDC/ETC could reflect the distribution of electron-accepting functional groups or electron-donating functional groups during composting [37]. kt/k0 is also correlated positively well with EAC/ETC but unremarkable (R = 0.740) (Figs. 3c and d). These lines of evidence suggested that the effectiveness of compost-derived HAs for enhancing bioreduction Cr(Ⅵ) in the Fe2O3-HA systems were positively associated with their EAC and distribution of electronaccepting functional groups. Specific ultraviolet absorbance at 254 nm (SUVA254) and aromatic H generally indicated the degree of condensation in the aromatic rings of HAs, which could indicate the quinonoid structures [38, 39]. kt/k0 showed a positive relation with SUVA254 (R = 0.842, p = 0.032) and aromatic H (R = 0.854, p = 0.031) (Figs. 3e and f). The results suggested that the quinonoid group was important functional groups, accelerating electron transfer between microorganism and Cr(Ⅵ). Therefore, the presence of compost-derived HAs had accelerated the reduction of Cr(Ⅵ) in the Fe2O3-HA systems compared to the control experiment, and the amount of electron-accepting functional groups (such as quinone/hydroquinone) played a major role during the process.

|
Download:
|
| Fig. 3. Ratios of kt/k0 vs. EAC (a) and EDC (b); ratios of kt/k0 vs. EAC/ETC (c) and EDC/ETC (d) of all compost-derived HAs; ratios of kt/k0 vs. SUVA254 (e) and aromatic H (f) of all compost-derived HAs in the Fe2O3-HA experiments. | |
Based on our results, the electron transfer between HA and Cr(Ⅵ) was extremely slow at pH 7. In other words, the reduction rate of Cr(Ⅵ) was determined by the rate of electron transfer between HA and Cr(Ⅵ) (rate-limiting step). Previous research found that HAs could be used as the electron shuttles to enhance electron transfer between microorganism and the poorly soluble, thereby mediating the dissimilatory Fe(Ⅲ) reduction [20, 40]. In this work, Fe(Ⅱ) adsorbed on the mineral surface had high reactivity toward the reduction of Cr(Ⅵ) [5, 6]. Therefore, Cr(Ⅵ) as the terminal electron acceptor was reduced to Cr(Ⅲ) completely by adsorbed Fe(Ⅱ) in the Fe2O3-HA systems. Thus, it was demonstrated that the iron mineral can enhance the electron transfer between HA and Cr(Ⅵ). Our results suggested that in the presence of HAs and iron mineral, MR-1 could provide electrons to compostderived HAs, which subsequently transferred the electrons to the iron mineral, and then the Fe(Ⅱ) derived from the reduction of iron mineral could rapidly reduce Cr(Ⅵ). Overall, the iron mineral played the important role in the process of HA-mediated Cr(Ⅵ) bioreduction.
Compost-derived HA is an efficient redox aggregate [8, 11, 39]. Here, the aforementioned results indicated that compost-derived HAs had a positive influence on Cr(Ⅵ) bioreduction (Fig. 4). The initial-stage compost-derived HAs could be used as the electron donator to reduce Cr(Ⅵ) because the polysaccharide in the inherent construction (Direct reduction). In addition, redox capacities of compost-derived HAs can accelerate the formation of dissimilatory iron mineral by accepting the electron from microorganisms, making the long-range and wide-range transmission of extracellular respiration electrons of microorganisms possible. In fact, EAC and the distribution of electron-accepting functional groups were positively correlated with kt/k0, with correlation coefficients (R) of 0.905 (p < 0.01) and 0.74, respectively. The quinonoid groups were important functional groups responsible for electron transfer between microorganism and Cr(Ⅵ). The iron mineral plays a crucial role in the process of HAmediated Cr(Ⅵ) bioreduction. Thus, compost-derived HAs mediated microbial dissimilatory iron mineral reduction, and the resulting Fe(Ⅱ) further enhanced Cr(Ⅵ) reduction, which was the most crucial mode of electron transfer in the Fe2O3-HA systems. This provides future insights into the effect of compost-derived HAs on the fate and transformation of Cr(Ⅵ). Overall, compost products had the potential to promote the reduction of Cr(Ⅵ), especially in areas containing abundant iron mineral.
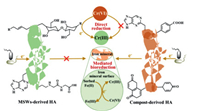
|
Download:
|
| Fig. 4. Schematic illustration of Cr(Ⅵ) reduction by compost-derived HA. | |
The authors declared that they have no any actual or potential conflict of interest to this work, including any financial, personal or other relationships with other people or organizations.
AcknowledgmentsThis work was supported by the National Key Research and Development Program of China (No. 2018YFC1800703) and the National Water Pollution Control and Management Technology Major Project of China (No. 2018ZX07110006).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.001.
| [1] |
J. Izbicki, M. Wright, W. Seymour, et al., Appl. Geochem. 63 (2015) 203-217. DOI:10.1016/j.apgeochem.2015.08.007 |
| [2] |
A. Zayed, N. Terry, Plant Soil 249 (2003) 139-156. DOI:10.1023/A:1022504826342 |
| [3] |
T. Rinehart, D. Schulze, R. Bricka, S. Bajt, E. Blatchley, J. Hazard. Mater. 52 (1997) 213-221. DOI:10.1016/S0304-3894(96)01808-0 |
| [4] |
D. Brose, B. James, Environ. Sci. Technol. 47 (2013) 12985-12991. DOI:10.1021/es401903s |
| [5] |
I. Buerge, S. Hug, Environ. Sci. Technol. 31 (1997) 1426-1432. DOI:10.1021/es960672i |
| [6] |
D. Hausladen, S. Fendorf, Environ. Sci. Technol. 51 (2017) 2058-2067. DOI:10.1021/acs.est.6b04039 |
| [7] |
H. Ji, Y. Zhu, J. Duan, W. Liu, D. Zhao, Chin. Chem. Lett. 30 (2019) 2163-2168. DOI:10.1016/j.cclet.2019.06.004 |
| [8] |
D. Said-Pullicino, F. Erriquens, G. Gigliotti, Bioresour. Technol. 98 (2007) 1822-1831. DOI:10.1016/j.biortech.2006.06.018 |
| [9] |
X. He, B. Xi, D. Cui, Y, et al., J. Hazard. Mater. 268 (2014) 256-263.
|
| [10] |
X. Xiao, B. Xi, X. He, et al., Chemosphere 222 (2019) 757-765. DOI:10.1016/j.chemosphere.2019.01.173 |
| [11] |
Y. Yuan, B. Xi, X. He, et al., J. Hazard. Mater. 339 (2017) 378-384. DOI:10.1016/j.jhazmat.2017.06.047 |
| [12] |
M. Aeschbacher, C. Graf, R. Schwarzenbach, M. Sander, Environ. Sci. Technol. 46 (2012) 4916-4926. DOI:10.1021/es300039h |
| [13] |
J. Zhang, L. Chen, H. Yin, et al., Environ. Pollut. 225 (2017) 86-92. DOI:10.1016/j.envpol.2017.03.047 |
| [14] |
G. Arslan, S. Edebali, E. Pehlivan, Desalination 255 (2010) 117-123. DOI:10.1016/j.desal.2010.01.006 |
| [15] |
A. Ohta, H. Kagi, H. Tsuno, M. Nomura, T. Okai, Geochem. J. 46 (2012) 409-420. DOI:10.2343/geochemj.2.0222 |
| [16] |
J. Zhang, H. Yin, L. Chen, F. Liu, H. Chen, Environ. Pollut. 237 (2018) 740-746. DOI:10.1016/j.envpol.2017.10.120 |
| [17] |
Q. Zhang, K. Amor, S. Galer, I. Thompson, D. Porcelli, WaterRes. 151 (2019) 98-109. |
| [18] |
M. Bishop, H. Dong, P. Glasser, et al., Geochim. Cosmochim. Acta 252 (2019) 88-106. DOI:10.1016/j.gca.2019.02.039 |
| [19] |
L. Klüpfel, A. Piepenbrock, A. Kappler, M. Sander, Nat. Geosci. 7 (2014) 195-200. DOI:10.1038/ngeo2084 |
| [20] |
X. Xiao, B. Xi, X. He, et al., Environ. Pollut. 253 (2019) 488-496. DOI:10.1016/j.envpol.2019.07.044 |
| [21] |
A. Zomeren, R. Comans, Environ. Sci. Technol. 41 (2007) 6755-6761. DOI:10.1021/es0709223 |
| [22] |
X. He, C. Yang, S. You, et al., Sci. Total Environ. 665 (2019) 920-928. DOI:10.1016/j.scitotenv.2019.02.164 |
| [23] |
W. Tan, B. Xi, G. Wang, et al., Environ. Sci. Technol. 51 (2017) 3176-3186. DOI:10.1021/acs.est.6b04131 |
| [24] |
B. Gu, J. Chen, Geochim. Cosmochim. Acta 67 (2003) 3575-3582. DOI:10.1016/S0016-7037(03)00162-5 |
| [25] |
Y. Gu, M. Yin, H. Zhang, Y. Wang, J. Shi, Spectrochim. Acta Part A 136 (2015) 1702-1709. DOI:10.1016/j.saa.2014.10.069 |
| [26] |
J. Gustafsson, I. Persson, A. Oromieh, et al., Environ. Sci. Technol. 48 (2014) 1753-1761. DOI:10.1021/es404557e |
| [27] |
T. Peretyazhko, G. Sposito, Geoderma 137 (2006) 140-146. DOI:10.1016/j.geoderma.2006.08.004 |
| [28] |
N. Kožuh, J. Stupar, B. Gorenc, Environ. Sci. Technol. 34 (2000) 112-119. DOI:10.1021/es981162m |
| [29] |
J. Ma, R. Del Vecchio, K. Golanoski, E. Boyle, N. Blough, Environ. Sci. Technol. 44 (2010) 5395-5402. DOI:10.1021/es100880q |
| [30] |
S. Lee, D. Kim, K. Kim, Chemosphere 194 (2018) 515-522. DOI:10.1016/j.chemosphere.2017.12.007 |
| [31] |
N. Mladenov, Y. Zheng, B. Simone, et al., Environ. Sci. Technol. 49 (2015) 10815-10824. DOI:10.1021/acs.est.5b01962 |
| [32] |
L. Wang, L. Ye, Y. Yu, C. Jing, Environ. Sci. Technol. 52 (2018) 5200-5207. |
| [33] |
X. Zhao, X. He, B. Xi, et al., Waste Manage. 70 (2017) 37-44. DOI:10.1016/j.wasman.2017.09.012 |
| [34] |
Y. Xie, G. Lu, H. Ye, et al., Chem. Geol. 475 (2017) 52-61. DOI:10.1016/j.chemgeo.2017.10.031 |
| [35] |
S. Huang, P. Chiang, J. Liu, et al., Chemosphere 87 (2012) 587-594. DOI:10.1016/j.chemosphere.2012.01.010 |
| [36] |
S. Chen, S. Huang, P. Chiang, et al., J. Hazard. Mater. 197 (2011) 337-344. DOI:10.1016/j.jhazmat.2011.09.091 |
| [37] |
S. Wu, G. Fang, Y. Wang, et al., Environ. Sci. Technol. 51 (2017) 9709-9717. DOI:10.1021/acs.est.7b01854 |
| [38] |
Z. Yang, M. Du, J. Jiang, Chemosphere 144 (2016) 902-908. DOI:10.1016/j.chemosphere.2015.09.037 |
| [39] |
M. Martoszek, J. Polak, W. Sułkowski, Chemosphere 73 (2008) 1465-1470. DOI:10.1016/j.chemosphere.2008.07.051 |
| [40] |
C. Poggenburg, R. Mikutta, M. Sander, et al., Chem. Geol. 447 (2016) 133-147. DOI:10.1016/j.chemgeo.2016.09.031 |
 2020, Vol. 31
2020, Vol. 31 

