b Research Center for Eco-Environmental Engineering, Dongguan University of Technology, Dongguan 523808, China
Antibiotics are now well recognized recalcitrant pollutants owing to their widespread use in human and animal diseases, poor reduction in traditional sewage treatment plants and pseudo persistence in water environment [1, 2]. Sulfamethoxazole (SMX) is one of the mostly used antibiotics for the treatment of urinary tract infections, respiratory infections, chronic bronchitis and meningitis. This antibiotic is frequently detected in wastewater effluents (100-2500 ng/L), surface waters (60-150 ng/L) and drinking waters (12 ng/L) [3, 4]. Although SMX is detected with trace concentration, the relative persistence will lead to its accumulation and result in ecotoxicological effects, such as drug resistance and carcinogenic activity [5, 6]. In addition, nearly 86% of ingested SMX is excreted as metabolites, not in its original form. Acetyl-sulfamethoxazole (Ac-SMX), the primary metabolite of SMX, is reported to be more persistent than its parent compound SMX. And the concentrations of Ac-SMX detected in wastewaters and surface waters are comparable to the parent compound SMX [7-10]. Thus, the elimination of SMX and its metabolites from wastewater is urgent and noteworthy.
Advanced oxidation processes (AOPs) have been studied to degrade SMX, such as ionizing radiation, ozonation, photocatalysis, photo-Fenton, heat-activated persulfate oxidation and electrochemical oxidation [11-15]. For example, the ionizing radiation and ozonation can effectively degrade SMX, while the mineralization of SMX is quite limited. Only 12.4% SMX mineralized after 1.5 kGy irradiation and the complete SMX abatement was achieved with just 10% of mineralization after 15 min ozonation [16, 17]. Photo-Fenton technology is limited by the requirement of strong acidity and generation of large sludge [9]. The SMX degradation efficiency of heat-activated persulfate oxidation increased with the increase of persulfate concentration, while this technology required extra treatment for the produced sulfuric acid wastewater [18].
Among these AOPs, electrochemical oxidation degradation appears as an available alternative due to its environmental compatibility, high energy efficiency and strong oxidation property [19-21]. The efficiency of electrochemical oxidation is mainly determined by anode materials, which can produce amounts of hydroxyl radicals (·OH)[22, 23]. PbO2 has been considered as an excellent anode because of good chemical stability and high oxygen evolution reaction overpotential [24, 25]. Doping rare earth elements (e.g., Er, Ce, and Gd) into PbO2 coating can significantly improve the efficiency of electrochemical oxidation [26, 27]. For example, the Ti/SnO2-Sb/Er-PbO2 electrode demonstrated the optimal performance for the oxidation of 4-chlorophenol compared to Ti/SnO2-Sb/Ce-PbO2 electrode, Ti/SnO2-Sb/La-PbO2 electrode and Ti/SnO2-Sb/Gd-PbO2 electrode [24]. However, it is unknow whether the SMX and Ac-SMX can be degraded by Ti/SnO2-Sb/Er-PbO2 electrode. The electrochemical degradation mechanism and toxicity variation of SMX and its metabolite have not been elucidated. Thus, it is necessary to have insights into the elimination of sulfamethoxazole and its metabolite by electrochemical degradation.
In this study, the electrochemical degradation of SMX and Ac-SMX was investigated by using Ti/SnO2-Sb/Er-PbO2 electrode. Affecting factors, such as current density, initial solution pH and inorganic ions, were investigated to analyze the kinetics and energy consumption of SMX and Ac-SMX. Degradation intermediates were identified to propose possible electrochemical degradation pathways of SMX and Ac-SMX in aqueous solution. Total organic carbon (TOC) and inorganic ions were measured. Toxicities of SMX, Ac-SMX and their intermediates were predicted using a quantitative structure-activity relationship (QSAR) model.
Electrochemical degradation experiments were conducted in an electrolytic cell. The Ti/SnO2-Sb/Er-PbO2 (anode) and stainless steel (cathode) were with the same dimension (50 mm × 50 mm × 1 mm), and the electrode distance was 1 cm. The life time of this anode was more than 1000 h and the electrode preparation is described in Supporting information. Initial concentrations of SMX (prepared with deionized water) and Ac-SMX (prepared with 1% acetonitrile cosolvent) were both 10 mg/L. Volume of electrolyte solution was 120 mL with 20 mmol/L Na2SO4 as supporting electrolyte. Five variables including current densities (4-18 mA/cm2), initial pH (3-11), Cl- concentrations (1-10 mmol/L), HCO3- concentrations (1-10 mmol/L) and NO3- concentrations (1-10 mmol/L) were investigated during the electrochemical degradation experiments. Degradation rate constants (k), half-lives (t1/2) and removal rates are illustrated in Tables S1 and S2 (Supporting information).
Fig. 1 exhibits the electrochemical degradation efficiencies of SMX and Ac-SMX under different current density. The k values of SMX were measured to be 0.058, 0.083, 0.139, 0.268 and 0.299 min-1 at the current density of 4, 6, 8, 10 and 12 mA/cm2, and the corresponding t1/2 values were 40.4, 28.6, 17.7, 10.4 and 9.2 min, respectively. The k values of Ac-SMX were 0.030, 0.043, 0.072, 0.073 and 0.090 min-1 at the current density of 10, 12, 14, 16 and 18 mA/cm2, and the corresponding t1/2 values were 77.2, 53.5, 34.0, 32.6 and 27.4 min, respectively. Compared to the k values (0.05-0.50 min-1) of SMX degradation at current density of 10-60 mA/cm2 using Ti/Ru0.3Ti0.7O2 anode, Ti/SnO2-Sb/Er-PbO2 anode showed higher degradation efficiency [28]. The degradation efficiencies of SMX and Ac-SMX were accelerated with the increasing current density, which may be due to the more ·OH production at higher current density [29, 30]. It also showed that the removal rates of SMX and Ac-SMX increased slowly when the current densities were higher than 10 and 14 mA/cm2, respectively, which may be owing to the lower rising tendency of ·OH production. Thus, 10 and 14 mA/cm2 were chosen as the optimal current density for the subsequence experiments of SMX and Ac-SMX to avoid energy waste. The removal rates of SMX and Ac-SMX under the optimal current density were 99.6% and 88.5% after 20 min and 30 min electrochemical reaction, respectively. These results illustrate that the Ac-SMX is more difficult to degrade than SMX. Therefore, investigating the electrochemical degradation behavior of Ac-SMX is essential to comprehensively analyze the risks related with SMX in the water bodies.

|
Download:
|
| Fig. 1. Effect of applied current density on the electrochemical degradation of SMX and Ac-SMX. Initial concentration: 10 mg/L, Na2SO4: 20 mmol/L, electrode distance: 1 cm, pH: unadjusted. | |
As shown in Fig. S1 (Supporting information), the effect of initial pH ranging from 3-11 on the electrochemical degradation efficiencies of SMX and Ac-SMX was investigated. For SMX, the k values increased from 0.183 to 0.457 min-1 with the pH decreasing from 11 to 3, and the corresponding removal rates increased from 97.8% to 99.9%. For Ac-SMX, the k values were 0.074, 0.078, 0.079, 0.087 and 0.094 min-1 at the pH of 11, 9, 7, 5 and 3, respectively. The corresponding removal rates slightly increased from 88.0% to 94.1%. Obviously, SMX and Ac-SMX were favorable to degrade under acidic condition. The species and hydration of target compound molecules are affected by the pH of solution [31]. Based on pKa of SMX (pKa1 = 1.8, pKa2 = 5.8) and Ac-SMX (pKa1 < 2, pKa2 = 5.1) [7], the molecular forms of SMX and Ac-SMX were dominant under acidic condition and the negatively charged forms were dominant under alkaline condition. The mass transfer coefficient of molecular form was higher than negatively charged form [32-34]. Thus, mass transfer efficiency under acidic condition was higher than that under alkaline condition, which resulted in higher k values at lower pH. In addition, concentration of ·OH decreased at alkaline condition due to its reaction with OH-, which also led to lower degradation efficiency [35, 36].
Fig. 2 illustrates the electrochemical degradation efficiencies of SMX and Ac-SMX under different concentrations of HCO3-, NO3- and Cl-. With the concentration of HCO3- increasing from 0 to 10 mmol/L, the k values of SMX and Ac-SMX were measured to be 0.244–0.277 and 0.064–0.069 min-1, respectively. The corresponding t1/2 values of SMX and Ac-SMX were determined to be 10.0-10.9 and 32.6-36.5 min, respectively. These results demonstrated that the addition of HCO3- had slight effect on the electrochemical degradation of SMX and Ac-SMX. HCO3- can significantly accelerate the degradation of oxcarbazepine, perfluorooctanoic acids and oxytetracycline, which may be due to the production of CO3·- via the reaction with ·OH [36-38]. While the HCO3- restrained the degradation efficiency of ciprofloxacin [33], norfloxacin [34] and abacavir [37] as the quencher of ·OH. These different effects were inferred that the transition between promotion and inhibition for the degradation was determined by the rate constant of CO3·- toward compound, and the value of carbonate rate constant for this transition was somewhere around 108 L mol-1 s-1 [39, 40].
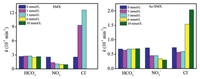
|
Download:
|
| Fig. 2. Degradation rate constants (k) of SMX and Ac-SMX at different anion concentrations. Applied current density: 10 mA/cm2 for SMX and 14 mA/cm2 for Ac-SMX, initial concentration: 10 mg/L, Na2SO4: 20 mmol/L, electrode distance: 2 cm, pH: unadjusted. Noting that the removal rate of SMX reached 99.0% in two minutes with the addition of 6 and 10 mmol/L Cl-, so the rate constants were not calculated. | |
For the concentration of NO3- of 0, 1, 3, 6 and 10 mmol/L, the k values of SMX were 0.254, 0.137, 0.108, 0.096 and 0.092 min-1, respectively. At the same NO3- concentration gradient, the k values of Ac-SMX were 0.072, 0.045, 0.045, 0.034 and 0.030 min-1, respectively. Compared to the sample without NO3-, the k value of SMX decreased by 63.8% and that of Ac-SMX decreased by 58.3% for the NO3- concentration of 10 mmol/L. The corresponding t1/2 values of SMX and Ac-SMX were 11.2-24.8 and 34.0-73.3 min, respectively. The removal rates of SMX decreased from 99.5% to 84.4% with the NO3- concentration increasing from 0 to 10 mmol/L, and those of Ac-SMX decreased from 88.5% to 62.5% as well. It was clearly that NO3- exerted an inhibitory performance on the degradation of SMX and Ac-SMX. This inhibitory effect of NO3- was also found in the electrochemical degradation of other organic pollutants, such as stavudine [41], lamivudine [42] and enrofloxacin [43]. The reason for the inhibition may be that NH3+ and NO2- (generated by the reduction of NO3-) can easily react with ·OH, which decreases the concentration of ·OH and results in the suppression phenomenon [30, 33, 44].
As for the effect of Cl-, the k values of SMX increased from 0.254 to 1.261 min-1 with the concentration of Cl- increasing from 0 to 3 mmol/L. The corresponding t1/2 values of SMX were 1.6-11.2 min. The removal rates of SMX reached 99.0% in two minutes with the addition of 6 and 10 mmol/L Cl-, so the rate constants were not calculated. The degradation efficiency of SMX was significantly accelerated with the addition of Cl-, which may be due to the generation of active chlorine species (e.g., Cl2, ClO-, ClO2-, Cl- and Cl·) via the reaction with ·OH or direct oxidation [45]. For Ac-SMX, the k values of Ac-SMX were 0.073, 0.057, 0.059, 0.154 and 0.204 min-1 in the presence of 0, 1, 3, 6 and 10 mmol/L Cl-, respectively. The corresponding t1/2 values of Ac-SMX were 31.4, 40.6, 38.3, 16.4 and 12.2 min. ·OH may be main oxidant for the Cl- concentration of 1 and 3 mmol/L, so the consumption of ·OH via reaction with Cl- led to an inhibitory effect. For the Cl- concentration of 6 and 10 mmol/L, the active chlorine species played a major role and showed a facilitation effect [16]. The different effects of SMX and Ac-SMX toward active chlorine species may be related with the phenylamino group as well as the reactivity and concentration of oxidants [46].
Electrochemical degradation products of SMX and Ac-SMX were determined by UPLC-MS/MS in the positive mode. Five major transformation products (TP286, TP270, TP112, TP110 and TP98) were identified for SMX and six transformation products (TP283, TP267, TP215, TP142, TP112 and TP98) were identified for Ac-SMX. Detailed information of these transformation products was shown in Table S3 (Supporting information). TP286 and TP270 were hydroxylated products of SMX. TP283 and TP267 were oxidation products of Ac-SMX. TP215, TP112 and TP98 were the products by the cleavage of S-N bond. TP100 was isoxazole ring-cleavage product.
In accordance with the discussions above, the possible degradation pathways of SMX (red line) and Ac-SMX (blue line) are proposed in Fig. 3. SMX was attacked by ·OH to form TP270, and further hydroxylated toTP286 [31]. Meanwhile, SMX generated the TP98 and S173 after the cleavage of S-N bond via the attack of ·OH. Then the amino of TP98 was oxidized to form the nitroso derivative TP112 and further oxidized to the nitro product S128 [47]. Meanwhile, the isoxazol ring of TP98 was attacked by ·OH to generate the ring-opening product TP100. Similar degradation pathway of Ac-SMX, including the cleavage of S-N bond and the transformation of TP98 were also indicated. Afterwards, product TP215 of Ac-SMX was transformed to the 4-amino benzene sulphinic acid S173 via the break of amide bond. Subsequently, the amino group and an oxygen atom of S173 were removed to generate TP142. Otherwise, the amide bond of Ac-SMX was broken, and further oxidized to nitroso derivative TP267 and nitro derivative TP283 [48]. Finally, these intermediates of SMX and Ac-SMX were mineralized to CO2, H2O and inorganic ions.

|
Download:
|
| Fig. 3. Proposed degradation pathways of SMX (red line) and Ac-SMX (blue line). Degradation products in dotted rectangle (marked "S") were not actually detected. | |
As aforementioned introduction, many techniques have been used to degrade SMX, such as ozonation and ionizing radiation. However, various organic intermediates were produced and complete mineralization was difficult during the degradation of SMX. Thus, it is significant to determine the mineralization of SMX by electrochemical degradation. As shown in Fig. S2 (Supporting information), the TOC removal of SMX was nearly 63.2% with 3 h electrochemical degradation. 22.4% nitrogen of SMX was transformed to NO3- and 98.8% sulfur was released as SO42-. Although the removal rate of SMX reached 99.6% within 20 min at 10 mA/cm2 current density, only 54.2% sulfur of SMX was recovered as SO42- and 14.9% nitrogen was transformed to NO3-, which implying that the total mineralization required longer reaction time or enhancing current density, which might be explained to the generation of refractory intermediates via the electrochemical degradation. However, compared with the TOC of SMX by electrochemical degradation using other anodes, Ti/SnO2-Sb/Er-PbO2 anode was still competitive. For example, the TOC removal of SMX was 28.6% after 2 h electrochemical degradation using Ti/Ru0.3Ti0.7O2 anode [28]. TOC and inorganic ions of Ac-SMX not measured because it was dissolved with acetonitrile cosolvent.
Although the degradation intermediates of SMX were identified in other studies, the toxicities of SMX, metabolite Ac-SMX and their intermediates have received little scrutiny to date. In this study, the toxicities of SMX, Ac-SMX and their intermediates were predicted based on QSAR model. The detailed values of toxicity are shown in Table S4 (Supporting information). As shown in Fig. 4, the chronic toxicity of metabolite Ac-SMX to fish, daphnia and green algae was higher than that of parent compound SMX, which indicated that it was noteworthy to investigate the treatment of Ac-SMX. The toxicities of SMX and Ac-SMX decreased after electrochemical degradation process. The toxicities of products with large molecular mass were higher than that of low molecular mass. TP142 possessed the lowest toxicity, and its lethal concentration (LC50), effective concentration (EC50) and chronic value (ChV) were hundreds of times higher than those of SMX and Ac-SMX. It was worth noting that the chronic toxicities of TP100 and TP98 to daphnia were higher than those of SMX and Ac-SMX, which indicated that assessing the potential risks to aquatic organisms was essential in view of some toxic intermediates.

|
Download:
|
| Fig. 4. Toxicity assessments for SMX, Ac-SMX and their intermediates. Very toxicity: LC50/EC50/ChV < 10°, toxicity: 101 > LC50/EC50/ChV > 10°, harmful: 102 > LC50/EC50/ChV > 101, not harmful: LC50/EC50/ChV > 102 [37]. | |
The economic feasibility of SMX and Ac-SMX degradation was valuated through electrical energy per order (EEO), and the calculation of EEO values is illustrated in Supporting information [49]. As exhibited in Table S5 (Supporting information), the EEO values of SMX were between 0.58 and 8.97 Wh/L, and those of Ac-SMX ranged from 6.88 Wh/L to 44.19 Wh/L. When the current density of SMX increased from 4 mA/cm2 to 12 mA/cm2, the lowest EEO value occurred at the current density of 10 mA/cm2 and determined to be 4.12 Wh/L. For Ac-SMX, the lowest EEO value occurred at the current density of 14 mA/cm2 and determined to be 21.15 Wh/L. These results were consistent with the choice of optimal current density of 10 mA/cm2 and 14 mA/cm2 for SMX and Ac-SMX, respectively. Notably, the EEO values significantly decreased in the presence of high concentration of Cl-, while the highest EEO values occurred in the presence of 10 mmol/L NO3-. These phenomena were mainly due to the different degradation rates of SMX and Ac-SMX under various conditions. It also indicated that inorganic ions ubiquitous in water environments were crucial for the practical application of electrochemical degradation process.
It was worth noting that the EEO values of metabolite Ac-SMX were higher than those of parent compound SMX, which can be explained by the lower degradation efficiency of Ac-SMX. This phenomenon further indicated that the metabolite Ac-SMX was more refractory than SMX. Compared with energy consumption values of other compounds, such as 0.87-2.29 Wh/L for stavudine [41], 0.099-2.988 Wh/L for naproxen [50], 3.3-62.1 Wh/L for benzophenone-3 [49] and 41.7-71.6 Wh/L for perfluorodecanoic acid [51], those of SMX and Ac-SMX were close to naproxen and benzophenone-3, but lower than that of perfluorodecanoic acid. The high energy consumption of perfluorodecanoic acid was ascribed to low degradation efficiency caused by the refractory C–F bond. As we known, energy consumption is affected by electrode activity, pollutant structure and reaction conditions, implying that optimizing reaction condition and improving electrode performance are effective way to reduce energy consumption in practical applications.
In conclusion, SMX and its metabolite Ac-SMX can be efficiently degraded by the Ti/SnO2-Sb/Er-PbO2 anode. The degradation of SMX and Ac-SMX is significantly accelerated in the presence of high concentration of Cl-, while NO3- exerts an inhibitory effect on the degradation of SMX and Ac-SMX. The degradation pathways of SMX and Ac-SMX mainly involve the cleavage of S–N bond, opening ring of isoxazole and nitration of amino group. The toxicities of SMX and Ac-SMX significantly decrease after electrochemical degradation. The metabolite Ac-SMX is more refractory and toxic compared with SMX, implying that dispose of metabolites should be taken into account when removing parent pharmaceuticals from wastewater. The process also demonstrates an effective removal of SMX and its metabolites from the hospital effluent.
Declaration of competing interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis study was financially supported by the National Science Fund for Distinguished Young Scholars (No. 51625801), the Guangdong Innovation Team Project for Colleges and Universities (No. 2016KCXTD023), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2017), the China Postdoctoral Science Foundation (No. 2018M643671).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.073.
| [1] |
W. Baran, E. Adamek, J. Ziemiańska, A. Sobczak, J. Hazard. Mater. 196 (2011) 1-15. DOI:10.1016/j.jhazmat.2011.08.082 |
| [2] |
S.P. Rong, Y.B. Sun, J.Z.H. Zhao, Chin. Chem. Lett. 25 (2014) 187-192. DOI:10.1016/j.cclet.2013.11.003 |
| [3] |
C. Wang, Y.B. Liu, T. Zhou, M.J. Huang, X.H. Wu, Chin. Chem. Lett. 30 (2019) 2231-2235. DOI:10.1016/j.cclet.2019.08.055 |
| [4] |
J. Lienert, K. Gudel, B.I. Escher, Environ. Sci. Technol. 41 (2007) 4471-4478. DOI:10.1021/es0627693 |
| [5] |
M.A. Gilliver, M. Bennett, M. Begon, S.M. Hazel, C.A. Hart, Nature 401 (1999) 233-234. DOI:10.1038/45724 |
| [6] |
A.K. Morris, R.G. Masterton, J. Antimicrob. Chemoth. 49 (2002) 7-10. DOI:10.1093/jac/49.1.7 |
| [7] |
F. Bonvin, R. Rutler, N. Chevre, J. Halder, T. Kohn, Environ. Sci. Technol. 45 (2011) 4702-4709. DOI:10.1021/es2003588 |
| [8] |
F. Bonvin, J. Omlin, R. Rutler, et al., Environ. Sci. Technol. 47 (2012) 6746-6755. |
| [9] |
M.D. Celiz, J. Tso, D.S. Aga, Environ. Toxicol. Chem. 28 (2009) 2473-2484. DOI:10.1897/09-173.1 |
| [10] |
A.G. Trovo, R.F.P. Nogueira, A. Aguera, et al., Water Res. 43 (2009) 3922-3931. DOI:10.1016/j.watres.2009.04.006 |
| [11] |
C. Baeza, D.R.U. Knappe, Water Res. 45 (2011) 4531-4543. DOI:10.1016/j.watres.2011.05.039 |
| [12] |
F.J. Beltrán, A. Aguinaco, J.F. Garcíya-Araya, A. Oropesa, Water Res. 42 (2008) 3799-3808. DOI:10.1016/j.watres.2008.07.019 |
| [13] |
O.S. Keen, K.G. Linden, Environ. Sci. Technol. 47 (2013) 13020-13030. DOI:10.1021/es402472x |
| [14] |
J.J. Zhan, M.F. Li, X.J. Zhang, et al., Chine. Chem. Lett. 31 (2020) 715-720. DOI:10.1016/j.cclet.2019.09.001 |
| [15] |
B. Wols, C. Hofman-Caris, D. Harmsen, E. Beerendonk, Water Res. 47 (2013) 5876-5888. DOI:10.1016/j.watres.2013.07.008 |
| [16] |
R. Zhuan, J.L. Wang, Sci. Total Environ. 668 (2019) 67-73. DOI:10.1016/j.scitotenv.2019.03.027 |
| [17] |
R.F. Dantas, S. Contreras, C. Sans, S. Esplugas, J. Hazard. Mater. 150 (2008) 790-794. DOI:10.1016/j.jhazmat.2007.05.034 |
| [18] |
H. Milh, B. Schoenaersb, A. Stesmansb, D. Cabooterc, R. Dewila, Chem. Eng. J. 379 (2020) 122234. DOI:10.1016/j.cej.2019.122234 |
| [19] |
H.J. Liang, P. Hua, Y.F. Zhou, et al., Chin. Chem. Lett. 30 (2019) 2245-2248. DOI:10.1016/j.cclet.2019.05.046 |
| [20] |
J. Li, J. Li, Z.K. Xiong, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 2139-2146. DOI:10.1016/j.cclet.2019.04.057 |
| [21] |
H. Lin, J.F. Niu, S.Y. Ding, L.Y. Zhang, Water Res. 46 (2012) 2281-2289. DOI:10.1016/j.watres.2012.01.053 |
| [22] |
D.B. Luo, S.H. Liu, K. Nakata, A. Fujishima, Chin. Chem. Lett. 30 (2019) 509-512. DOI:10.1016/j.cclet.2018.06.010 |
| [23] |
Q.F. Zhuo, S.B. Deng, B. Yang, et al., Electrochim. Acta 77 (2012) 17-20. DOI:10.1016/j.electacta.2012.04.145 |
| [24] |
J.T. Kong, S.Y. Shi, L.C. Kong, X.P. Zhu, J.R. Ni, Electrochim. Acta 53 (2007) 2048-2054. DOI:10.1016/j.electacta.2007.09.003 |
| [25] |
S. Song, L.Y. Zhan, Z.Q. He, et al., J. Hazard. Mater. 175 (2010) 614-621. DOI:10.1016/j.jhazmat.2009.10.051 |
| [26] |
Z.S. Xu, Y.X. Yu, H. Liu, J.F. Niu, Sci. Total Environ. 579 (2016) 1600-1607. |
| [27] |
L.C. Zhang, L. Xu, J. He, J.J. Zhang, Electrochim. Acta 117 (2014) 192-201. DOI:10.1016/j.electacta.2013.11.117 |
| [28] |
S. Hussain, J.R. Steter, S. Gul, A.J. Motheo, J. Environ. Manage. 201 (2017) 153-162. DOI:10.1016/j.jenvman.2017.06.043 |
| [29] |
M. Skoumal, R.M. Rodriguez, P.L. Cabot, et al., Electrochim. Acta 54 (2009) 2077-2085. DOI:10.1016/j.electacta.2008.07.014 |
| [30] |
C. Wang, J.F. Niu, L.F. Yin, J.X. Huang, L.A. Hou, Chem. Eng. J. 346 (2018) 662-671. DOI:10.1016/j.cej.2018.03.159 |
| [31] |
H. Lin, J.F. Niu, J.L. Xu, Y. Li, Y.H. Pan, Electrochim. Acta 97 (2013) 167-174. DOI:10.1016/j.electacta.2013.03.019 |
| [32] |
I.B. Rivas-Ortiz, G. Cruz-González, A.M. Lastre-Acosta, et al., J. Radioanal. Nucl. Chem. 314 (2017) 1-11. DOI:10.1007/s10967-017-5353-4 |
| [33] |
Z.B. Guo, S.N. Zhu, Y.F. Zhao, H. Cao, F.L. Liu, Environ. Sci. Pollut. Res. 22 (2015) 15772-15780. DOI:10.1007/s11356-015-4715-0 |
| [34] |
M. Sayed, M. Ismail, S. Khan, S. Tabassum, H.M. Khan, Environ. Technol. 37 (2016) 590-602. DOI:10.1080/09593330.2015.1075597 |
| [35] |
Y.Q. Liu, X.X. He, X.D. Duan, Y.S. Fu, Water Res. 95 (2016) 195-204. DOI:10.1016/j.watres.2016.03.011 |
| [36] |
T.C. Liu, K. Yin, C.B. Liu, J.M. Luo, J. Crittenden, Water Res. 147 (2018) 204-213. DOI:10.1016/j.watres.2018.10.007 |
| [37] |
C.Z. Zhou, Y.P. Wang, J. Chen, et al., Chemosphere 225 (2019) 304-310. DOI:10.1016/j.chemosphere.2019.03.036 |
| [38] |
L.P. Thi, H.T. Do, Y.C. Lee, S.L. Lo, Chem. Eng. J. 221 (2013) 258-263. DOI:10.1016/j.cej.2013.01.084 |
| [39] |
Amina, X.Y. Si, K. Wu, Y.B. Si, B. Yousaf, Chem. Eng. J. 353 (2018) 80-91. DOI:10.1016/j.cej.2018.07.078 |
| [40] |
D. Vione, S. Khanra, S.C. Manc, et al., Water Res. 43 (2009) 4718-4728. DOI:10.1016/j.watres.2009.07.032 |
| [41] |
C.Z. Zhou, Y.P. Wang, J. Chen, J.F. Niu, Environ. Int. 133 (2019) 105157. DOI:10.1016/j.envint.2019.105157 |
| [42] |
Y.P. Wang, C.Z. Zhou, J. Chen, Z.M. Fu, J.F. Niu, Electroanal. Chem. 848 (2019) 113314. DOI:10.1016/j.jelechem.2019.113314 |
| [43] |
C. Wang, L.F. Yin, Z.S. Xu, J.F. Niu, L.A. Hou, Chem. Eng. J. 326 (2017) 911-920. DOI:10.1016/j.cej.2017.06.038 |
| [44] |
S.D. Jojoa-Sierra, J. Silva-Agredo, E. Herrera-Calderon, R.A. Torres-Palma, Sci. Total Environ. 575 (2017) 1228-1238. DOI:10.1016/j.scitotenv.2016.09.201 |
| [45] |
A. Thiam, E. Brillas, F. Centellas, P.L. Cabot, I. Sirés, Electrochim. Acta 173 (2015) 523-533. DOI:10.1016/j.electacta.2015.05.085 |
| [46] |
R.C. Zhang, P.Z. Sun, T.H. Boyer, L. Zhao, C.H. Huang, Environ. Sci. Technol. 49 (2014) 3056-3066. |
| [47] |
J.S. Du, W.Q. Guo, H.Z. Wang, et al., Water Res. 138 (2018) 323-332. DOI:10.1016/j.watres.2017.12.046 |
| [48] |
G.F. Liu, X.C. Li, B.J. Han, et al., J. Hazard. Mater. 322 (2017) 461-46. DOI:10.1016/j.jhazmat.2016.09.062 |
| [49] |
C.Z. Zhou, Y.P. Wang, J. Chen, J.F. Niu, Sci. Total Environ. 688 (2019) 75-82. DOI:10.1016/j.scitotenv.2019.06.197 |
| [50] |
L. Xu, X. Ma, J.F. Niu, J. Chen, C.Z. Zhou, J. Hazard. Mater. 379 (2019) 120692. DOI:10.1016/j.jhazmat.2019.05.085 |
| [51] |
H. Lin, J.F. Niu, J.L. Xu, et al., Environ. Sci. Technol. 47 (2013) 13039-13046. DOI:10.1021/es4034414 |
 2020, Vol. 31
2020, Vol. 31 

