b School of Engineering, Edith Cowan University, 270 Joondalup Drive, Perth WA 6027, Australia;
c China General Nuclear Power Hongda Environmental Science and Technology Co., Ltd., Jinan 250000, China;
d Dongying Municipal Bureau of Marine Development and Fisheries, Dongying Municipal Marine and Fishery Environmental Monitoring Center, Dongying 257091, China;
e School of Chemical Engineering and Advanced Materials, The University of Adelaide, Adelaide SA 5005, Australia
The ever-increasing environmental issues and global energy shortage have significantly affected human life and the surrounding environment [1-4]. Semiconductor-based photocatalysis, utilizing photo-driven redox reactions, has demonstrated as one of the most effective strategies to address these problems because it is clean, safe, and of no secondary pollution [5-7]. In the exploration process of robust and efficient photocatalysts, graphitic carbon nitride (g-C3N4) stands out because of the typical two-dimensional layered conjugate structure, good thermochemical stability, suitable band gap structure, and easy preparation [8-10]. However, bulk g-C3N4 has some inherent drawbacks such as sluggish separation of photo-generated carriers, small specific surface areas (SSA), limited accessible reactive sites, and low absorption capacity for visible light, which dramatically urge further improvements [11, 12].
To address these issues, a number of strategies have been developed, including elements doping [13], heterojunctions [14], and morphology regulation [15]. The development of photocatalysts with porous structures was regarded as an effective strategy to modulate the reactive sites, facilitate the mass transfer, and ease the photo-generated electron-hole recombination [16]. Hard-templating and soft-templating routes were popular approaches to construct porous g-C3N4 to improve its photocatalytic performance [17]. For example, Wang et al. [18] synthesized g-C3N4 with a mesoporous structure by employing SBA-15 as hard-templating synthesis. However, these hard templates need to be removed by extreme hazardous reagents such as NH4HF2 and HF, which are environmentally unfriendly and demanding a time-consuming process [19]. The soft templates are easier and safer to be removed, while the pore channels of g-C3N4 tend to be collapsed during polymerization at high temperatures [20]. Therefore, exploring a nontoxic and simple method to fabricate porous-rich g-C3N4 still remains a great challenge.
Previously reported works suggest that ultrathin porous g-C3N4 can be obtained via the polymerization of the acidified nitrogenrich precursors [21, 22]. For example, Dong et al. [23] fabricated g-C3N4 with a thin porous platelet-like structure via the polymerization of HCl-acidified melamine. The g-C3N4 derived from HCl-acidified melamine exhibited a larger SSA than that of the sample derived from pure melamine. Zhang and coworkers [24] prepared the acidified g-C3N4 by pretreating HCl with melamine. The increased porosity and SSA for the g-C3N4 sample were attributed to the evaporation of Cl- during polymerization of the precursors. Nevertheless, such a protocol only results in a slight increased SSA, which still demands a significant further improvement. The direct polymerization of urea can produce porous g-C3N4 with a high SSA, while the extremely low yield dramatically limits its scale application. In this respect, Zhang et al. [25] demonstrated that urea can serve as a green bubble template to enhance the yield and to enlarge the SSA. Moreover, the SSA and photocatalytic performance of bubble template derived-g-C3N4 can be modulated by changing the ratio of urea and dicyandiamide. Bearing the merits of both pre-acidified nitrogen-rich precursors and bubble template, it is expected to achieve more interesting results when combining these two green and simple strategies.
Herein, we demonstrated that highly porous g-C3N4 (HCl-CNU-X) can be feasibly prepared via co-polymerization of the preacidified melamine and urea. Transmission electron microscopy (TEM) and nitrogen sorption results indicated that the prepared HCl-CNU-X possessed a porous structure and large SSA. In addition, HCl-CNU-X exhibited excellent activity toward both photocatalytic degradation and hydrogen production. This work is expected to provide a new paradigm for designing efficient and robust photocatalysts for environmental remediation and water splitting.
The HCl-CNU-X photocatalyst was fabricated by the copolymerization of the pre-acidified melamine and urea, as illustrated in Scheme 1. In the first step, melamine was dispersed in HCl solution under vigorously stirring to form melamine hydrochloride. Then, HCl-CNU-X was obtained by annealing the mixture of melamine hydrochloride and urea at 550 ℃. The detailed fabrication method is provided in Supporting information.

|
Download:
|
| Scheme 1. The synthesis procedure of HCl-CNU-X. | |
The microstructures of the prepared samples were first examined by scanning electron microscopy (SEM) and TEM images. The pristine g-C3N4 (Figs. S1a and c in Supporting information) showed an irregular compact bulk structure. After the pre-acidification process, a thinner platelet-like structure with numerous mesoporous was formed in HCl-CN (Figs. S1b and d in Supporting information), suggesting that the pre-acidification process affected the growth orientation [23]. With both preacidification and bubble template modification, HCl-CNU-3 exhibited a structure with in-plane pores (Figs. 1a and b). The results revealed that the pre-acidification of melamine and the introduction of a bubble template promote the formation of the porous structure.

|
Download:
|
| Fig. 1. (a) SEM and (b) TEM of HCl-CNU-3. (c) XRD of g-C3N4, HCl-CN, and HCl-CNU-X. (d) Nitrogen sorption isotherms of g-C3N4, HCl-CN, and HCl-CNU-3. | |
In the X-ray diffraction (XRD) patterns of the g-C3N4, HCl-CN, and HCl-CNU-X (Fig. 1c), all samples exhibited two typical distinct diffraction peaks at approximately 13.1° and 27.3°, which were ascribed to (100) in-plane packing motifs and the stacking of (002) interlayer, respectively [26]. However, compared with stacked g-C3N4, the peaks of HCl-CN and HCl-CNU-X samples were much weaker, possibly due to the less ordered stacking of tri-s-triazine units and the existence of the disordered crystal structure [27]. The pre-acidification of melamine could weaken the force between layers to make g-C3N4 thinner and inhibit the crystal growth of g-C3N4 [23]. In addition, urea can be decomposed into CO2 and H2O at a lower temperature, enabling it as a bubble-template to boost the formation of porous precursor structure and disordered crystal [28].
The Fourier transform infrared spectroscopy (FT-IR) spectra in Fig. S2a (Supporting information) indicated that all photocatalysts exhibited similar absorption vibration peaks at around 800 cm-1, resulting from the tri-s-triazine units as the main structure of the samples [27]. In addition, a wide acidified absorption band was found at 1250-1650 cm-1 stemmed from the presence of C—N heterocycle [29], while those broad peaks (3000-3700 cm-1) were from N—H stretching [30]. The above results indicated that the polymerization of the acidified precursors and urea did not destroy the main framework.
To investigate the textural properties, nitrogen sorption tests (Fig. 1d) was performed. All g-C3N4, HCl-CN, and HCl-CNU-3 showed a type Ⅳ isotherms and H3 hysteresis loop, indicating that the samples possessed a mesoporous structure [31]. In addition, the pore diameter of stacked g-C3N4 and HCl-CN were distributed in the range of 2-50 nm, while HCl-CNU-3 was located between 2 nm and 100 nm (Fig. S2b in Supporting information) with the presence of mesopores and a few of macropores. The pore volume of HCl-CNU-3 was also larger than g-C3N4 and HCl-CN. The detailed nitrogen sorption parameters are listed in Table S1 (Supporting information). Noteworthy, the SSA of the HCl-CNU-3 composite was 49.8 m2/g, which was approximately 10 times larger than that of g-C3N4. The porous structure and large SSA of HCl-CNU-3 are expected to provide more adsorption centers and reactive sites to boost the photocatalytic performance.
The surface compositions and chemical states of g-C3N4, HCl-CN, and HCl-CNU-3 were further examined by X-ray photoelectron spectroscopy (XPS). The survey spectra of all samples (Fig. S3a in Supporting information) indicated that they contained C, N, and O elements. No residual Cl- (the binding energy of Cl 2p is 198.7 eV) was detected in HCl-CN and HCl-CNU-3, because Cl- transformed into HCl upon polymerization of the precursors. The release of HCl is favorable for the formation of porosity and the increase of SSA in HCl-CNU-3 [24]. In the C 1s spectrum (Fig. S3b in Supporting information), the two main peaks at 287.9 eV and 284.6 eV corresponding to N—C=N and C—C, respectively [32]. For the N 1s spectrum (Fig. S3c in Supporting information), the three peaks at 398.4, 399.8, and 400.9 eV were assigned to C—N=C, N–(C)3, and C-N-H, respectively [33]. In terms of O 1s (Fig. S3d in Supporting information), the small peak located at 533.4 eV was owing to the appearance of C = O, and the large peak at 531.9 eV was due to -OH [34].
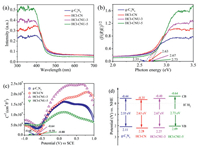
Although FT-IR and XPS show nearly no difference in the samples, the alteration of the optical property on the HCl-CN and HCl-CNU-3 was observed. As shown in Fig. 2a, the UV–vis DRS of HCl-CN and HCl-CNU-3 exhibited a slight redshift in comparison to g-C3N4, suggesting that the acidification of precursors is favorable for the improved optical absorption. Moreover, the estimated band gap energies of g-C3N4, HCl-CN, HCl-CNU-3 and HCl-CNU-5 were 2.55, 2.63, 2.67 and 2.73 eV (Fig. 2b), respectively. The conduction band (CB) was determined by the linear region intercepts of Mott–Schottky curves (Fig. 2c), and the flat band potentials of g-C3N4, HCl-CN, HCl-CNU-3 and HCl-CNU-5 were estimated to be ‒0.68, ‒0.59, ‒0.64 and ‒0.88 V (versus SCE, ‒0.44, ‒0.35, ‒0.40 and ‒0.64 V versus NHE), respectively [25]. According to the band-gap data, the valence bands (VB) were 2.11, 2.28, 2.27 and 2.09 V (versus NHE) for g-C3N4, HCl-CN, HCl-CNU-3 and HCl-CNU-5, respectively. The schematic band gap structures of the g-C3N4, HCl-CN, HCl-CNU-3 and HCl-CNU-5 are depicted in Fig. 2d. The VB of HCl-CN and HCl-CNU-3 were more positive than that of g-C3N4 and HCl-5, suggesting that the two samples might possess stronger oxidation ability.
|
Download:
|
| Fig. 2. (a) UV–vis DRS absorption spectrums. (b) The band-gap determination plots. (c) Mott–Schottky conduction band curves. (d) Schematic band-gap structure of the g-C3N4, HCl-CN, HCl-CNU-3 and HCl-CNU-5. | |
The recombination efficiency of photoinduced carriers is a key factor in governing the photocatalytic performance, which can be evaluated by photoluminescence (PL) [35]. As depicted in Fig. S4a (Supporting information), HCl-CNU-3 exhibited a dramatically decreased peak intensity (around 470 nm) than that of g-C3N4 and HCl-CN. The weakened peak in HCl-CNU-3 suggest the lowered recombination rate of charge carriers. The charge mobility was further characterized by electrochemical impedance spectra (EIS). In general, the smaller diameter of the sample corresponding to the more efficient separation of charge-carriers [36]. HCl-CNU-3 exhibited the smallest arc radius among three samples (Fig. S4b in Supporting information), indicating the efficient separation of photoinduced carriers. The EIS result was also consistent with the photoluminescence spectra. The improved carriers' mobility and inhibited recombination of photogenerated carriers can be ascribed to the formed porous structure and the exposure of more reactive sites.
The photocatalytic performances of g-C3N4, HCl-CN, and HCl-CNU-X were investigated by the degradation of tetracycline hydrochloride (TC). Prior to switching on the light, the adsorption-desorption tests were performed in dark for 40 min. As shown in Fig. S5a (Supporting information), all samples achieved their adsorption-desorption equilibrium within approximately 10 min. In the photocatalytic degradation process, HCl-CNU-3 exhibited the highest degradation efficiency of 72% in 60 min (Fig. 3a), and the first-order rate constant of HCl-CNU-3 was 7.5 times of stacked g-C3N4 (Fig. S5b in Supporting information), even higher or compatible with the reported photocatalysts (Table S2 in Supporting information). The enhanced performance was attributed to the formed porous structure in HCl-CN-3, which can provide more accessible active sites, boost the electron-hole separation, and inhibit carriers' recombination. In addition, the VB edge of HCl-CNU-3 (2.27 V) was deeper than that of HCl-CNU-5 (2.09 V), suggesting that HCl-CNU-3 possessed the stronger oxidation ability than that of HCl-CNU-5 (Fig. 2d). HCl-CNU-3 also maintained an excellent stability of photodegradation efficiency after four cycles (Fig. 3b). Besides, HCl-CNU-5 exhibited the highest hydrogen production rate (32.5 μmol/h) which was about 4.2 times of stacked g-C3N4 (Fig. 3c) among all the samples. The improved H2 performance can be attributed to that the CB of HCl-CNU-5 (-0.64 V) was more negative than the HCl-CNU-3 (‒0.40 V) (Fig. 2d). The value was also higher or compatible with the reported g-C3N4-based nanostructures photocatalysts (Table S3 in Supporting information). The H2 evolution stability of HCl-CNU-5 was evaluated within four cycles. As shown in Fig. 3d, HCl-CNU-5 exhibited a quite stable performance in the first two runs, while a slightly decreased generation rate of H2 was observed in the third cycle owing to the fast consumption of triethanolamine (TEOA) When 5 mL TEOA was added into the solution before the fourth run, the original H2 evolution rate was recovered. The rapid consumption of TEOA also provided an indirect evidence for the highly efficient H2 evolution.

|
Download:
|
| Fig. 3. (a) photocatalytic degradation curves of g-C3N4, HCl-CN, and HCl-CNU-X. (b) The degradation stability of HCl-CNU-3 and (c) H2 evolution efficiency of g-C3N4, HCl-CN, and HCl-CNU-X loaded 3.0 wt% Pt as cocatalyst. (d) The H2 evolution stability of HCl-CNU-5 loaded 3.0 wt% Pt as cocatalyst. | |
To shed light on the enhanced photocatalytic mechanism, trapping experiments were performed (Fig. S6 in Supporting information). In the photocatalytic system, iso-propyl alcohol (IPA), TEOA, and N2 were selected as ·OH, h+, and O2·- scavengers, respectively [37-39]. The photocatalytic degradation performance decreased slightly after the addition of IPA. In contrast, when the TEOA and N2 were added into the solution, the TC degradation efficiency decreased to 42% and 25%, respectively. Therefore, both O2·- and h+ played a major role in the system.
Based on the above discussion, a plausible photocatalytic reaction mechanism for HCl-CNU-3 is proposed in Scheme 2. Upon visible light irradiations, the electrons were excited to the CB and leaving holes on the VB. Thereafter, electrons on the CB reduced O2 to produce O2·- on reactive sites or reduce water into hydrogen with the aid of Pt. On the VB, TC could be directly degraded by h+ due to its strong oxidation ability.

|
Download:
|
| Scheme 2. Schematic illustration of the proposed mechanism of HCl-CNU-3 sample. | |
In summary, porous g-C3N4 photocatalysts were prepared via the co-polymerization of the acidified melamine and a green bubble template (urea). HCl-CNU-3 rendered a dramatically enhanced photocatalytic TC degradation rate of about 7.5 times of g-C3N4. Moreover, HCl-CNU-5 (derived from 25 g urea as the bubble template) exhibited a H2 production rate of 32.5 μmol/h, 4.2 times higher than that of g-C3N4. The enhanced photocatalytic activity was ascribed to the formation of the porous structure, which dramatically promoted the separation of carriers and facilitated efficient electron transfer. The photocatalytic mechanism studies showed that the main active components were O2·- and h+ in the degradation of TC. This work provides a new paradigm of robust porous photocatalysts for environmental remediation and water splitting.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors thank the National Science and Technology Major Project (No. 2016ZX05040003); Shuaijun Wang thanks the China Scholarship Council Scholarship (No. 201806450064)
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2020.08.003.
| [1] |
W.J. Ong, L.L. Tan, Y.H. Ng, S.T. Yong, S.P. Chai, Chem. Rev. 116 (2016) 7159-7329. DOI:10.1021/acs.chemrev.6b00075 |
| [2] |
B. Jiang, Q. Niu, C. Li, N. Oturan, M.A. Oturan, Appl. Catal. B 272 (2020) 119002. DOI:10.1016/j.apcatb.2020.119002 |
| [3] |
P. Deng, H. Ren, Q. Jiao, Vacuum 169 (2019) 108882. DOI:10.1016/j.vacuum.2019.108882 |
| [4] |
S. Li, J. Chen, S. Hu, et al., Inorg. Chem. Front. 7 (2020) 529-541. DOI:10.1039/C9QI01201J |
| [5] |
Z. Mo, X. Zhu, Z. Jiang, et al., Appl. Catal. B 256 (2019) 117854. DOI:10.1016/j.apcatb.2019.117854 |
| [6] |
X. Li, J. Xiong, X. Gao, et al., J. Hazard. Mater. 387 (2019) 121690. |
| [7] |
Q. Hao, Y. Huang, D. Chen, et al., Chin. J. Catal. 41 (2020) 249-258. DOI:10.1016/S1872-2067(19)63450-9 |
| [8] |
J. Xiong, X. Li, J. Huang, et al., Appl. Catal. B 266 (2020) 118602. DOI:10.1016/j.apcatb.2020.118602 |
| [9] |
M. Ding, J. Zhou, H. Yang, et al., Chin. Chem. Lett. 31 (2020) 71-76. DOI:10.1016/j.cclet.2019.05.029 |
| [10] |
S. Wang, L. Chen, X. Zhao, et al., Appl. Catal. B 278 (2020) 119312. DOI:10.1016/j.apcatb.2020.119312 |
| [11] |
Q. Hao, G. Jia, W. Wei, et al., Nano Res. 13 (2020) 1-20. DOI:10.1007/s12274-019-2585-3 |
| [12] |
Z. Gao, K. Chen, L. Wang, et al., Appl. Catal. B 268 (2020) 118462. DOI:10.1016/j.apcatb.2019.118462 |
| [13] |
G. Zhang, M. Zhang, X. Ye, et al., Adv. Mater. 26 (2014) 805-809. DOI:10.1002/adma.201303611 |
| [14] |
S. Kuecken, A. Acharjya, L. Zhi, et al., Chem. Commun. 53 (2017) 5854-5857. DOI:10.1039/C7CC01827D |
| [15] |
Y. Cui, Y. Tang, X. Wang, Mater. Lett. 161 (2015) 197-200. DOI:10.1016/j.matlet.2015.08.106 |
| [16] |
S. Zhang, Y. Liu, P. Gu, et al., Appl. Catal. B 248 (2019) 1-10. DOI:10.1016/j.apcatb.2019.02.008 |
| [17] |
X.H. Li, X. Wang, M. Antonietti, Chem. Sci. 3 (2012) 2170-2174. DOI:10.1039/c2sc20289a |
| [18] |
X. Chen, J. Zhang, X. Fu, M. Antonietti, X. Wang, J. Am. Chem. Soc. 131 (2009) 11658-11659. DOI:10.1021/ja903923s |
| [19] |
B. Long, Y. Zheng, L. Lin, et al., J. Mater. Chem. A 5 (2017) 16179-16188. DOI:10.1039/C6TA09802A |
| [20] |
J. Lin, Z. Pan, X. Wang, ACS Sustain. Chem. Eng. 2 (2013) 353-358. DOI:10.1021/sc4004295 |
| [21] |
B. Zhu, G. Xu, X. Li, et al., J. Mater. Res. 34 (2019) 3462-3473. DOI:10.1557/jmr.2019.294 |
| [22] |
S. Zhao, J. Fang, Y. Wang, et al., J. Colloid Interf. Sci. 561 (2020) 601-608. DOI:10.1016/j.jcis.2019.11.035 |
| [23] |
G. Dong, L. Zhang, J. Mater. Chem. 22 (2012) 1160-1166. DOI:10.1039/C1JM14312C |
| [24] |
X.S. Zhang, J.Y. Hu, H. Jiang, Chem. Eng. J. 256 (2014) 230-237. DOI:10.1016/j.cej.2014.07.012 |
| [25] |
M. Zhang, J. Xu, R. Zong, Y. Zhu, Appl. Catal. B 147 (2014) 229-235. DOI:10.1016/j.apcatb.2013.09.002 |
| [26] |
S. Wang, Q. Yan, P. Dong, et al., Appl. Phys. A 124 (20118) 416. |
| [27] |
M. Zhu, S. Kim, L. Mao, et al., J. Am. Chem. Soc. 139 (2017) 13234-13242. DOI:10.1021/jacs.7b08416 |
| [28] |
G. Zhang, J. Zhang, M. Zhang, X. Wang, J. Mater. Chem. 22 (2012) 8083-8091. DOI:10.1039/c2jm00097k |
| [29] |
S. Wang, H. Zhao, X. Zhao, et al., Chem. Eng. J. 381 (2020) 122593. DOI:10.1016/j.cej.2019.122593 |
| [30] |
X. Shi, M. Fujitsuka, S. Kim, T. Majima, Small 14 (2018) 1703277. DOI:10.1002/smll.201703277 |
| [31] |
S. Li, S. Hu, K. Xu, et al., Nanomaterials 7 (2017) 22. DOI:10.3390/nano7010022 |
| [32] |
S. Wang, F. He, X. Zhao, et al., Appl. Catal. B 257 (2019) 117931. DOI:10.1016/j.apcatb.2019.117931 |
| [33] |
Y. Liu, H. Zhang, J. Ke, et al., Appl. Catal. B 228 (2018) 64-74. DOI:10.1016/j.apcatb.2018.01.067 |
| [34] |
X. Li, D. Xie, H. Park, et al., Nanoscale 5 (2013) 1945-1948. DOI:10.1039/c2nr33795a |
| [35] |
S. Li, J. Chen, S. Hu, et al., Chem. Eng. J. 402 (2020) 126165. DOI:10.1016/j.cej.2020.126165 |
| [36] |
F. Yu, L. Wang, Q. Xing, et al., Chin. Chem. Lett. 31 (2020) 1648-1653. DOI:10.1016/j.cclet.2019.08.020 |
| [37] |
X. Ma, K. Chen, B. Niu, et al., Chin. J. Catal. 41 (2020) 1535-1543. DOI:10.1016/S1872-2067(19)63486-8 |
| [38] |
S. Shanavas, S. Mohana Roopan, A. Priyadharsan, et al., Appl. Catal. B 255 (2019) 117758. DOI:10.1016/j.apcatb.2019.117758 |
| [39] |
J. Liu, H. Xu, Y. Xu, et al., Appl. Catal. B 207 (2017) 429-437. DOI:10.1016/j.apcatb.2017.01.071 |
 2020, Vol. 31
2020, Vol. 31 

