b College of Hydraulic and Hydroelectric Engineering, Sichuan University, Chengdu 610065, China;
c Sino-German Centre for Water and Health Research, Sichuan University, Chengdu 610065, China
Due to the increased resistance of microorganisms to antibiotics and their latent risks to humans and ecosystems, antibiotics present in water bodies have attracted widespread attention recently [1]. Sulfamethoxazole (SMX), one of the sulfa antibiotics, has been wildly used in veterinary disease treatment, livestock and poultry breeding [2, 3]. Thus, with the rapid development of medical and animal husbandry, SMX has been extensively detected in sewage, surface water and groundwater [4, 5]. Although the detected SMX concentration is at a relatively low level, the cumulative effect caused by its low biodegradability would have a non-negligible impact on aquatic ecosystems [6]. Therefore, an efficient method is needed for removing and controlling SMX in various water bodies.
Advanced oxidation processes (AOPs) have been widely investigated and utilized as an efficient water treatment technology, it can efficiently degrade and mineralize refractory organic pollutants in the aquatic environment [7]. Generally, reactive oxygen species (ROS) such as sulfate radical (SO4·-), hydroxyl radical (HO·), superoxide radical (O2·-) and singlet oxygen (1O2) are generated in the AOPs [8]. Traditional Fenton reaction is based on a homogeneous Fe2+/H2O2 system to generate HO·, which has the disadvantages of pH limitation and large amounts of sludge [9, 10]. Compared with HO·, SO4·- has a higher oxidation potential (ESO4·- = 2.5-3.1 V; EHO· = 1.8-2.7 V) under neutral conditions, a longer half-life, a higher selectivity for organics as well as a wide range of effective pH [11-13]. For a long time, heating [14], electrolysis [15], ultraviolet (UV) radiation, transition metal ions and their oxide counterparts have been commonly used to activate persulfate (PDS) and peroxymonosulfate (PMS) or degrade pollutants directly [15-18]. However, the above activation methods have some drawbacks, including high cost and metal ion leaching [19]. Therefore, researchers gradually turned their attention to metal-free materials for PMS/PDS activation.
Meanwhile, with the development of research, the non-radical pathway with 1O2 as ROS has been found in the field of PDS activation. Zhou et al. showed that benzoquinone could activate PMS to produce 1O2 to degrade SMX [20]. In addition, 1O2 was demonstrated to be present in the degradation of 2, 4-dichlorophenol (2, 4-DCP) by PDS activation with carbon nanotubes (CNT) [21]. Studies showed that 1O2 present in the AOPs exhibited the selective response to different organic pollutants, but it has broad applicability for various environments contained natural free radical scavengers [22]. Nowadays, metalfree carbon materials such as carbon nanotube, grapheme, activated carbon, and g-C3N4 have been used as PDS/PMS activators for environmental remediation or applied in hydrogen evolution [21, 23-25]. Meanwhile, the study showed that similarities existed in these metal-free carbon materials with different non-radical mechanisms, such as large surface area and excellent electron transfer ability [26, 27]. However, the specific non-radical mechanisms of carbon materials in PDS activation needed to be further verified.
Acetylene black is the carbon black obtained by the decomposition and refining of by-product gas during the pyrolysis of calcium carbide or naphtha (naphtha). It has strong electrical conductivity and liquid absorbency. Herein, in this work, we used AB as a metal-free catalyst to activate PDS, and SMX was selected as the model contaminant. Then, a series of control experiments were conducted to test the catalytic ability of AB. Besides, the key parameters affecting the SMX degradation efficiency (i.e., AB dosage, PDS dosage, pH value and initial SMX concentration) were explored (Supporting information). Moreover, the predominant ROS was detected by scavenging tests. Finally, a possible mechanism of SMX degradation in AB/PDS system was proposed.
The surface morphology of the fresh and used AB was characterized by scanning electron microscopy (SEM). As shown in Figs. 1a and b, no obvious morphological changes of AB could be observed after the reaction. The SEM images reveal the unique spatial chain structure composed of spherical particles with crosslinking property, and the particle diameter was about 1 μm. Meanwhile, both the fresh and used AB displayed a porous surface, which was considered to facilitate the diffusion of reaction substrates and the exposure of active sites [28].

|
Download:
|
| Fig. 1. SEM image of (a) the fresh and (b) the used AB. | |
X-ray diffraction (XRD) was used to determine the crystalline structure of AB. As displayed in Fig. 2a, the broad reflection peak at 2θ = 26.5° was corresponding to the (002) plane, while the weak diffraction peak at 2θ = 43.5° was corresponding to the (100) plane. These two peaks proved the existence of sp2carbon bonded graphite crystals in the material and enabled AB to show specific graphite properties [29]. Raman spectra are widely used to evaluate the degree of graphitization of carbon atoms in materials. Raman analysis of AB (Fig. 2b) accords with the typical spectral characteristics of carbonaceous materials. The fresh and used AB both exhibited two peaks at 1346 and 1580 cm-1, which could be assigned to the D-band and G-band, respectively.Previous literature had shown that the D-band represented the structural defect and disorder induction of carbon, and G-band could be related to the stretching vibration mode of graphite crystals [30]. The intensity ratio of D band to G band (ID/IG) is an important indicator toevaluate the structural disorder of carbon materials. The ID/IG value indicates the degree of defect/disorder and a low degree of graphitization. In our case, the ID/IG corresponding to the fresh AB and used AB were 1.04 and 1.03, indicating that no significant change happened in the graphitization degree of AB after the reaction. Meanwhile, the higher graphitization degree is favorable for promoting the electron transfer between the oxidant and the carbon catalyst to speed up the catalytic reaction efficiency [28].

|
Download:
|
| Fig. 2. (a) XRD patterns of the fresh AB and used AB, (b) Raman spectra, (c) N2 adsorption-desorption isotherms and (d) pore size distributions of AB. | |
N2 adsorption-desorption measurements were employed to evaluate the pore texture properties of AB, and the results are illustrated in Figs. 2c and d. The Brunauer-Emmett-Teller (BET) surface area and pore volume of AB derived from isothermal curve were 398.12 m2/g and 0.33 cm3/g, respectively. According to IUPAC classification, the material presented typical Ⅳ isotherm with a usual H3 hysteresis loop [31]. The steep uptake of adsorption at lower relative pressure (P/P0 > 0.4) could be attributed to the existence of micropores, and the hysteresis of isothermal curve corresponded to the capillary condensation in porous. A prominent peak at 3 nm was observed in the pore size distribution (PSD) of the sample, and most of the pore diameter was concentrated within 4 nm, which further confirmed the microporous structure of the material.
To evaluate the catalytic activity of catalyst, control experiments (PDS alone, AB alone and AB/PDS system) were set up under the same condition (i.e., SMX concentration = 5 μmol/L, AB dosage = 0.3 g/L, PDS dosage = 50 μmol/L and pH 7.0). As illustrated in Fig. 3a, a low SMX degradation efficiency (4%) was observed by PDS alone system within 15 min, implying the weak self-decomposition of PDS in the absence of a catalyst. Meanwhile, when only AB was added to the aqueous solution, about 23% of SMX was adsorbed by AB. The slight adsorption removal could be attributed to the large specific surface area of AB. However, SMX rapidly reached complete degradation in the presence of AB and PDS simultaneously. The result indicates AB could be used as a favorable catalyst to activate PDS for SMX degradation. Besides, two traditional carbon catalysts (graphite and biochar) were employed to verify the superior degradation properties of AB/PDS system (Fig. 3b). SMX degradation efficiency was only 11% in both graphite/PDS and biochar/PDS systems, which was far lower than that of AB/PDS system. Thus, compared with other traditional catalysts, AB showed better capacity on activating PDS.

|
Download:
|
| Fig. 3. (a) Degradation of SMX under various systems and (b) comparison with other conventional carbocatalysts for PDS activation. Experiment conditions: [SMX]0 = 5 μmol/L, [catalyst]0 = 0.3 g/L, [PDS]0 = 50 μmol/L, initial pH 7.0, T = 25 ℃. | |
Besides, pseudo-first-order model (Eq. 1) was adopted to evaluate the catalytic reaction kinetic rate constants of SMX degradation. As seen in Fig. S7a (Supporting information), the kobs sequences of different systems were as follows, PDS alone (0.004 min-1) < AB alone (0.023 min-1) < AB/PDS (0.328 min-1). The kobs in AB/PDS system was 82 times and 14 times of that in the PDS alone and AB alone systems, which reflected the excellent catalytic effect of AB. Besides, the kobs value of 0.010 min-1 was observed in graphite/PDS and biochar/PDS systems (Fig. S7b in Supporting information), which further indicated the superiority of AB/PDS system.

|
(1) |
where [SMX] is the concentration (μmol/L) of SMX at the instance, [SMX]0 is the initial SMX concentration, kobs (min-1) is the pseudo-first-order rate constant of SMX degradation and t is the reaction time.
A series of quenching tests were performed to identify the ROS in AB/PDS system. It has been thoroughly demonstrated in the literature that ethanol (EtOH) has high reactivity with both SO4·- and HO· (k2 (EtOH, SO4·-) = 1.6-7.7 × 107 L mol-1 s-1, k2 (EtOH, HO·) = 1.2-2.8 × 109 L mol-1 s-1), while the reaction rate of TBA with HO· is 1000-fold faster than that with SO4·- (k2 (TBA, SO4·-) = 4.0-9.1 × 105 L mol-1 s-1, k2 (TBA, HO·) = 3.8-7.6 × 108 L mol-1 s-1) [32]. Therefore, in this study, EtOH was chosen to scavenge both SO4·- and HO·, while TBA was chosen as the scavenger for HO·. Seen from Fig. 4a, the degradation efficiency of SMX remained unchanged in the presence of 10 mmol/L EtOH and TBA, and the kobs value was 0.311 and 0.291 min-1, respectively (Fig. S8a in Supporting information). The result demonstrates that little SO4·- and HO· were produced in AB/PDS system. Furthermore, the hydrophilicity of alcohols would lead to their inability to react with surface adsorbed radicals according to the previous research [33]. Thus, dimethyl sulfoxide (DMSO) was selected to quench surfacebounded SO4·- and HO· [34]. The result shows no inhibiting effect was observed during the SMX degradation process, and the corresponding kobs were 0.310 min-1 (Fig. S8a in Supporting information), indicating that surface-bounded radicals did not play a role in the reaction system.

|
Download:
|
| Fig. 4. (a) Effects of different scavengers (EtOH, TBA, DMSO, FFA and L-His) at the concentration of 10 mmol/L and (b) AB after ozonation on the degradation of SMX. Experiment conditions: [SMX]0 = 5 μmol/L, [AB]0 = 0.3 g/L, [PDS]0 = 50 μmol/L, initial pH 7.0, T = 25 ℃. | |
In addition, FFA and L-His were proverbial scavengers for 1O2 ((k2 (FFA, 1O2) = 1.2 × 108 L mol-1 s-1), (k2 (L-His, 1O2) = 3.2 × 107 L mol-1 s-1)) [21, 35]. To investigate the non-radical pathway in AB/PDS system, a moderate concentration (10 mmol/L) of FFA and L-His were employed to confirm the presence of 1O2. From Fig. 4a, the SMX degradation efficiency decreased sharply from 100% to 30% with the addition of FFA. Meanwhile, the kobs value decreased from 0.328 to 0.029 min-1. Besides, when L-His was added, AB/PDS system showed low SMX degradation efficiency (34%) as well as kobs value (0.038 min-1). Both two phenomena manifested that 1O2 was dominantly responsible for the degradation of SMX by AB/PDS system. It was worth noting that, although FFA and L-His were highly reactive with free radicals, the previous conclusion pointed out that AB/PDS system produced a few of them [36]. Thus, it could be concluded that FFA and L-His existed only as quenchers of 1O2 in the established system.
Based on the above results, a probable catalytic mechanism of PDS activation by AB for SMX degradation was proposed. First, SMX molecules would be adsorbed on the surface of AB. Subsequently, the reductive functional groups on the surface of AB would transfer electrons to oxidant (PDS) to generate 1O2, which would react with SMX adsorbed on or near the AB surface rapidly to achieve high degradation efficiency. Moreover, to verify the role of reductive functional groups in the process of 1O2 generation, we used ozone oxidized AB to degrade SMX under the same condition. As illustrated in Fig. 4b, the degradation efficiency of SMX (3%) was significantly inhibited under the catalysis of ozone oxidized AB. The kobs value declined from 0.328 min-1 to 0.003 min-1 (Fig. S8b in Supporting information), indicating that the reducing functional groups on AB surface played a crucial role in the activation of PDS.
To make our conjecture convincing, FTIR was conducted to reveal the molecular structure changes. The FTIR spectra of the fresh and used AB were presented in Fig. S9 (Supporting information). Specifically, the bands locating at 3440 and 1640 cm-1 could be ascribed to the O—H, C—C functional groups, respectively [29]. The peak at 2920 cm-1 could be assigned to the antisymmetric stretching vibration of methylene. Additionally, the peak appearing at 1040 cm-1 might corresponding to the vibration of C—O—C [37]. It should be noted that compared with the fresh AB, the peak corresponding to the C-H deformational vibrations of CH3 groups at 1385 cm-1 was significantly weakened in the used AB [38]. The weakening of the peak after the reaction corresponded to the consumption of reducing functional groups, thus verifying the key role of reducing functional groups in PDS activation.
To test the potential of AB/PDS system for use in practice, the feasibility of the catalyst in actual waterbody should be evaluated. Owing to a series of micropollutants present in the real waterbody, the degradation ability of AB/PDS system for different contaminants were supposed to be studied. Herein, we selected four kinds of micropollutants, including bisphenol S (BPS), 2, 4-dichlorophenol (2, 4-DCP), p-nitrophenol (PNP) and diclofenac (DCF) at the concentration of 5 μmol/L. As shown in Fig. 5a, the non-negligible removal efficiencies of the four pollutants were observed in the presence of AB alone. Within 15 min, the adsorption removal of AB on BPS, 2, 4-DCP, PNP and DCF were 65%, 48%, 32% and 56%, respectively. More obviously, when AB and PDS were added together, BPS and 2, 4-DCP could be completely degraded within 3 min (Fig. 5b). By comparison, the time required for the complete degradation of PNP and DCF in AB/PDS system was longer, but only 10 min. All in all, the efficient degradation efficiencies of different pollutants proved that AB has good applicability as a catalyst.

|
Download:
|
| Fig. 5. (a) Adsorption of various pollutants by AB alone, (b) degradation efficiencies of various pollutants in AB/PDS system, (c) degradation of SMX by AB/PDS system with the presence of actual waterbody and (d) effects of actual water temperature on the degradation of SMX. Experiment conditions: [contaminant]0 = 5 μmol/L, [AB]0 = 0.3 g/L, [PDS]0 = 50 μmol/L, initial pH = 7.0, T = 25 ℃. | |
Besides, the same experiment for SMX degradation was conducted in tap water and river water. The tap water and river water samples were collected from Sichuan University and Funan river, respectively. Fig. 5c shows that compared with deionized water, the degradation efficiency of SMX in tap water and river water had an inevitable decrease. Specifically, after 15 min, 81% of SMX was degraded in tap water, while the degradation efficiency reached 67% in river water. The kobs values of tap water and river water were 0.130 and 0.090 min-1, respectively (Fig. S10a in Supporting information). The synthetical effects, such as adsorption on the catalyst surface, of the co-exciting ions (e.g., NO3-, Cl-, HCO3-, and HPO42-) and natural organic matters existed in real water samples, might be the possible reason for the relatively low SMX degradation efficiency.
Due to the difference of actual water temperature in nature, five temperature gradients (15 ℃, 20 ℃, 25 ℃, 30 ℃, 35 ℃) were set up to explore the effect of reaction temperature on the degradation efficiency of SMX. The result illustrated in Fig. 5d shows that SMX could be completely degraded in the temperature range of 15-35 ℃. Meanwhile, the kobs value was also elevated from 0.234 min-1 at 15 ℃ to 0.630 min-1 at 35 ℃ (Fig. S10b in Supporting information), indicating that heat activation could increase the SMX degradation efficiency. Furthermore, according to the Arrhenius equation (Eq. 2), the activation energy (Ea, kJ/mol) for the SMX degradation in AB/PDS system was calculated as 35.94 kJ/mol (R2 = 0.95). Compared with the Ea value of diffusioncontrolled reactions (in the range of 10-13 kJ/mol), the Ea value of SMX degradation implied that the apparent reaction efficiency of SMX degradation was mainly controlled by the efficiency of natural chemical reactions on the catalyst surface rather than the efficiency of mass transfer [39].
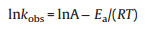
|
(2) |
Where kobs were the reaction rate constant, A was the preexponential factor, R was the general gas constant (8.314 J mol-1 K-1), and T was the kelvin temperature (K).
In this study, AB was used as PDS activator to degrade SMX for the first time. AB/PDS system exhibited excellent performance for SMX degradation compared with AB alone, PDS alone, graphite/PDS, and biochar/PDS systems. Then, the effects of catalyst dosage, PDS dosage, initial pH value, and initial SMX concentration on the degradation of SMX were studied in detail. SMX could be degraded completely within 15 min under the conditions of AB dosage = 0.3 g/L, PDS dosage = 50 μmol/L, initial SMX concentration = 5 μmol/L and initial pH 7.0. Meanwhile, common anions (Cl-, HCO3-, and NO3-) exhibited little effect on the degradation of SMX in AB/PDS system, indicating that AB has good applicability as a catalyst. Besides, the practical application tests revealed that AB/PDS system demonstrated satisfactory performance in the actual water body. Moreover, based on the investigations of scavenger experiments, 1O2 was determined to be the main active species in AB/PDS system. In general, as a metal-free catalyst, AB has a broad prospect in activating PDS for SMX degradation.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThe authors would like to acknowledge the financial support from National Natural Science Foundation of China (No. 51878423), Sichuan Science and Technology Program: Key Research and Development Program (Nos. 2019YFG0314, 2017SZ0180, 2019YFG0324) and the Major Scientific and Technological Special Program of Sichuan Province, China (2018SZDZX0027).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.08.008.
| [1] |
A. Yazdanbakhsh, A. Eslami, M. Massoudinejad, M. Avazpour, Chem. Eng. J. 380 (2020) 122497. DOI:10.1016/j.cej.2019.122497 |
| [2] |
J.C. Murillo-Sierra, E. Ruiz-Ruiz, L. Hinojosa-Reyes, et al., Catal. Today 313 (2018) 175-181. DOI:10.1016/j.cattod.2017.11.003 |
| [3] |
R. Yin, W. Guo, H. Wang, et al., Chem. Eng. J. 334 (2018) 2539-2546. DOI:10.1016/j.cej.2017.11.174 |
| [4] |
D. Archundia, C. Duwig, F. Lehembre, et al., Sci.TotalEnviron. 576 (2017) 671-682. |
| [5] |
M. Qiao, G.G. Ying, A.C. Singer, Y.G. Zhu, Environ. Int. 110 (2018) 160-172. DOI:10.1016/j.envint.2017.10.016 |
| [6] |
J. Liang, X. Xu, W. Qamar Zaman, et al., Chem. Eng. J. 375 (2019) 121908. DOI:10.1016/j.cej.2019.121908 |
| [7] |
Y. Yang, J.J. Pignatello, J. Ma, W.A. Mitch, Environ. Sci. Technol. 48 (2014) 2344-2351. DOI:10.1021/es404118q |
| [8] |
Y. Li, X. Zhao, Y. Yan, et al., Chem. Eng. J. 376 (2019) 121302. DOI:10.1016/j.cej.2019.03.178 |
| [9] |
E. Neyens, J. Baeyens, J. Hazard. Mater. 98 (2003) 33-50. DOI:10.1016/S0304-3894(02)00282-0 |
| [10] |
S. Wacławek, H.V. Lutze, K. Grübel, et al., Chem. Eng. J. 330 (2017) 44-62. DOI:10.1016/j.cej.2017.07.132 |
| [11] |
P. Hu, M. Long, Appl. Catal. B:Environ. 181 (2016) 103-117. DOI:10.1016/j.apcatb.2015.07.024 |
| [12] |
Z. Wu, Y. Wang, Z. Xiong, et al., Appl. Catal. B:Environ. 277 (2020) 119136. DOI:10.1016/j.apcatb.2020.119136 |
| [13] |
H. Zhang, Q. Ji, L. Lai, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 1129-1132. DOI:10.1016/j.cclet.2019.01.025 |
| [14] |
Y. Ji, Y. Shi, W. Dong, et al., Chem. Eng. J. 298 (2016) 225-233. DOI:10.1016/j.cej.2016.04.028 |
| [15] |
M. Zhu, L. Zhang, S. Liu, Chin. Chem. Lett. 31 (2020) 1961-1965. DOI:10.1016/j.cclet.2020.01.017 |
| [16] |
D. He, Y. Cheng, Y. Zeng, et al., Chemosphere 240 (2020) 124979. DOI:10.1016/j.chemosphere.2019.124979 |
| [17] |
L. Jianchang, G. Ming, H. Zheng, G. Changsheng, Chem. Eng. J. 389 (2020) 124456. DOI:10.1016/j.cej.2020.124456 |
| [18] |
J. Li, Y. Li, Z. Xiong, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 2139-2146. DOI:10.1016/j.cclet.2019.04.057 |
| [19] |
M. Hannah, S. Ben, S. Andre, C. Deirdre, D. Raf, Chem. Eng. J. 379 (2020) 122234. DOI:10.1016/j.cej.2019.122234 |
| [20] |
Y. Zhou, J. Jiang, Y. Gao, et al., Environ. Sci. Technol. 49 (2015) 12941-12950. DOI:10.1021/acs.est.5b03595 |
| [21] |
C. Xin, H. Guo, Y. Zhang, W. Xiao, L. Yang, Water Res. 113 (2017) 80-88. DOI:10.1016/j.watres.2017.02.016 |
| [22] |
X. Duan, H. Sun, Z. Shao, S. Wang, Appl. Catal. B:Environ. 224 (2018) 973-982. DOI:10.1016/j.apcatb.2017.11.051 |
| [23] |
P. Marta, D. Goran, B.T. Pedro, L.F. José, M.T.S. Adrián, Chem. Eng. J. 369 (2019) 223-232. DOI:10.1016/j.cej.2019.02.211 |
| [24] |
C. Yao, Y. Zhang, M. Du, X. Du, S. Huang, Chem. Eng. J. 362 (2019) 262-268. DOI:10.1016/j.cej.2019.01.040 |
| [25] |
F. Yu, L. Wang, Q. Xing, et al., Chin. Chem. Lett. 31 (2020) 1648-1653. DOI:10.1016/j.cclet.2019.08.020 |
| [26] |
M. Zhang, R. Luo, C. Wang, et al., J. Mater. Chem. A 7 (2019) 12547-12555. DOI:10.1039/C9TA02931A |
| [27] |
G. Liu, J. Zhou, W. Zhao, Z. Ao, T. An, Chin. Chem. Lett. 31 (2020) 1966-1969. DOI:10.1016/j.cclet.2019.12.023 |
| [28] |
N. Wang, W. Ma, Z. Ren, et al., J. Mater. Chem. A 6 (2018) 884-895. DOI:10.1039/C7TA08472B |
| [29] |
J. Tang, J. Yang, X. Zhou, J. Xie, G. Chen, Microporous Mesoporous Mater. 193 (2014) 54-60. DOI:10.1016/j.micromeso.2014.03.020 |
| [30] |
W. Ma, N. Wang, T. Tong, et al., Carbon 137 (2018) 291-303. DOI:10.1016/j.carbon.2018.05.039 |
| [31] |
M. Kruk, M. Jaroniec, Chem. Mater. 13 (2001) 3169-3183. DOI:10.1021/cm0101069 |
| [32] |
S. Lu, G. Wang, S. Chen, et al., J. Hazard Mater. 353 (2018) 401-409. DOI:10.1016/j.jhazmat.2018.04.021 |
| [33] |
Y. Yao, C. Lian, G. Wu, et al., Appl. Catal. B:Environ. 219 (2017) 563-571. DOI:10.1016/j.apcatb.2017.07.064 |
| [34] |
J. Yu, L. Tang, Y. Pang, et al., Appl. Catal. B:Environ. 260 (2020) 118160. DOI:10.1016/j.apcatb.2019.118160 |
| [35] |
Y. Wang, D. Cao, X. Zhao, Chem. Eng. J. 328 (2017) 1112-1121. DOI:10.1016/j.cej.2017.07.042 |
| [36] |
Y. Li, J. Li, Y. Pan, et al., Chem. Eng. J. 384 (2020) 123361. DOI:10.1016/j.cej.2019.123361 |
| [37] |
M. Varga, T. Izak, V. Vretenar, Carbon 111 (2017) 54-61. DOI:10.1016/j.carbon.2016.09.064 |
| [38] |
J. Chłopek, A. Morawska-Chochół, C. Paluszkiewicz, J. Mol. Struct. 875 (2008) 101-107. DOI:10.1016/j.molstruc.2007.04.021 |
| [39] |
Y. Feng, D. Wu, Y. Deng, T. Zhang, K. Shih, Environ. Sci. Technol. 50 (2016) 3119-3127. DOI:10.1021/acs.est.5b05974 |
 2020, Vol. 31
2020, Vol. 31 

