Just recently, metal organic frameworks (MOFs) have attracted increasing attention as potential photocatalysts [1, 2]. For the photocatalytic activity of MOFs, many researches focused on the application of CO2 reduction, water decomposition, organic pollutant degradation and Cr(Ⅵ) reduction due to its outstanding characteristics of large specific surface area, adjustable pore size and good topological structure [1-4]. The construction of heterojunctions between MOFs and semiconductors is increasingly used to improve their photocatalytic activity under visible light or even sunlight. Lately, some silver based semiconductors are considered to be promising visible responsive photocatalysts. Among them, Ag/Ag3PO4 is considered as a highly efficient co-existence photocatalyst, in which Ag particles can not only produce surface plasmon resonance (SPR) effect or electron mediator to boost the absorption of visible light, but also avoid retard the light corrosion of Ag3PO4 [5]. Cui et al. constructed Ag/Ag3PO4/RGO heterojunction to improve the performance of photocatalytic organic contaminants degradation [6]. Majid et al. reported that the construction of HKUST-1@Ag/Ag3PO4 can enhance the performance of Ponceau BS degradation under visible light [7]. Cr(Ⅵ), as a typical heavy metal, is a common pollutant discharged from textile manufacturing, steel manufacturing, leather tanning, and other industrial fields [8]. The photocatalytic reduction of Cr(Ⅵ) to Cr(Ⅲ) is considered as a feasible strategy to reduce its toxicity and easily eliminate it from environment [9, 10].
Herein, MIL-125-NH2 was selected to combine Ag/Ag3PO4 to construct Ag/Ag3PO4/MIL-125-NH2 composites for effective Cr(Ⅵ) reduction under visible light. In addition, the stability and mechanism of Ag/Ag3PO4/MIL-125-NH2 Z-scheme heterojunction were also discussed.
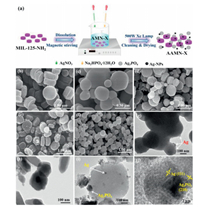
The disk-like MIL-125-NH2 was solvothermally synthesized according to a previous report with slight modification (Supporting information) [11]. The binary Ag3PO4/MIL-125-NH2 (AMN-X, like AMN-40, 100, 120, 150) were prepared through ion-exchange-solution method (Supporting information). The ternary Ag/Ag3PO4/MIL-125-NH2 (AAMN-X, like AAMN-40, 100, 120, 150) were fabricated via photo chemical reduction deposition approach (Fig. 1a). Taking AAMN-120 for an example, the obtained AMN-120 composite was added to 100 mL distilled water, and the suspension was ultrasonicated for 10 min. The system was then illuminated using a 500 W Xe light (Beijing Aulight Co., Ltd.) for 60 min to reduce partial Ag+ ions on the surface of AMN-120 into Ag0 element. Finally, the powder was collected by filtration, cleaned with ultra-pure water for several times, and dried oven at 60 ℃ overnight. Similarly, other AAMN-X composites with different proportions were prepared following the fabrication procedure of AAMN-120. For the sake of comparison, the Ag/Ag3PO4 nanoparticles was prepared according to the identical method of AAMN-120 fabrication method without adding MIL-125-NH2.
|
Download:
|
| Fig. 1. (a) Schematic formation of the AAMN-X composites; SEM images of (b) MIL-125-NH2, (c) Ag3PO4, (d) AMN-120, (e) AAMN-120 and (f) Ag/Ag3PO4; TEM images of (g) Ag/Ag3PO4, (h) MIL-125-NH2 and (i) AAMN-120; (j) HRTEM of AAMN-120. | |
The scanning electron microscope (SEM) (Figs. 1b-e) and transmission electron microscope (TEM) image of MIL-125-NH2 (Fig. 1h) revealed that the sphere-like structure Ag3PO4 were uniformly dispersed on the disk-like MIL-125-NH2. The Ag/Ag3PO4 maintained the morphology of the pristine Ag3PO4 (Fig. 1f), in which the minor Ag0 nanoparticles (NPs) were distributed over the surface of Ag3PO4 (TEM as illustrated in Fig. 1g). The formation of Ag/Ag3PO4/MIL-125-NH2 (like AAMN-120) was further affirmed by both TEM and high resolution TEM (HRTEM) (Figs. 1i and j), in which the lattice fringe of 0.236 nm and 0.266 nm observed in HRTEM corresponded to the (111) and (210) facets of Ag0 and Ag3PO4 nanoparticles, respectively [12]. The element mapping results (Fig. S3 in Supporting information) of AAMN-120 revealed the uniform distribution of Ag, O, P, Ti, N, and C elements, further confirming the successful construction of Ag/Ag3PO4/MIL- 125-NH2.
The AAMN-X were further characterized by powder X-ray diffraction (PXRD) and Fourier transform infrared (FTIR) (Figs. S4a and S4b in Supporting information). The PXRD peaks of MIL-125- NH2 were in good agreement with those reported in the former literature [13]. The simulated XRD pattern of Ag3PO4 and the standard pattern of cubic Ag3PO4 matched well, indicating that Ag3PO4 has been successfully prepared (JCPDS No. 97-000-1530) (Fig. S5 in Supporting information). The Ag/Ag3PO4 depicted the identical PXRD patterns to the pure Ag3PO4, suggesting that the incorporation of Ag particles exerted minor effect on the phase structure and crystallite size of Ag3PO4. The characteristic peaks of Ag and Ag3PO4 were observed in the PXRD patterns of AAMN-X particles, and the characteristic peaks of Ag3PO4 were also observed in AMN-X, indicating that AMN-X and AAMN-X were successfully prepared (Figs. S4a and S6 in Supporting information) [14]. AMN-X and AAMN-X displayed typical vibrational bands of carboxylate groups (1400-1700 cm-1) in MIL-125(Ti)-NH2 [15]. The peaks around 1040 and 550 cm-1 were related to the asymmetric stretching vibration modes of P—O—P and bending vibration of O=P—O of Ag3PO4 [16]. In their UV-vis diffuse reflection spectra (UV-vis DRS), the absorption edges of MIL-125- NH2 and Ag3PO4 were determined as ca. 465 and 532 nm with the Eg as ca. 2.67 and 2.33 eV, respectively (Figs. S4c and S4d in Supporting information). The energy gap of AAMN-120 (Eg = 2.55 eV) was smaller than that of AMN-120 (Eg = 2.71 eV) owing to the surface plasmon absorption effect of Ag0 (Fig. S4d), also indicating that the AAMN-120 composite can be excited by visible light [17].
X-ray photoelectron spectroscopy (XPS) (Fig. S7 in Supporting information) was used to further verify the surface composition and chemical state of AAMN-120. The typical peaks of elements in both Ag3PO4 and MIL-125-NH2 were presented simultaneously in the spectrum of AAMN-120 (Fig. S7a). The Ag 3d, Ti 2p, P 2p, C 1s, N 1s, and O 1s spectrum of AAMN-120 were analyzed detailly in Supporting information. The spectrum of Ag 3d in Ag/Ag3PO4 (Fig. S8 in Supporting information) can be delimited totwo parts at about 367.99/374.10 eV and 367.06/371.45 eV, corresponding to Ag0 and Ag+, respectively [18, 19].
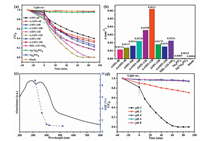
The photocatalytic Cr(Ⅵ) decontamination activities of different photocatalysts upon the illumination of visible light were explored at pH 2.0, as shown in Figs. 2 a and b. It was found that the increase of MIL-125-NH2 content in binary AMN-40, AMN-100 and AMN-120 as well as ternary AAMN-40, AAMN-100, and AAMN-120 resulted in increasing photocatalytic efficiencies (Fig. 2a) and improved reaction rates (k value) fitted by pseudo-first-orderkinetic model (Fig. 2b). It was worth noting that AAMN-X possessed superior photocatalytic activities to AMN-X, probably due tothat the existence of Ag NPs could promote the separation of photogenerated electrons and holes. As demonstrated in Fig. 2b, the reaction rate (k value) of AAMN-120 was 0.0621 min-1, 1.73, 2.77 and 124.2 times higher than those of AMN-120, MIL-125-NH2 and Ag3PO4, respectively. However, the excessive introduction of MIL-125-NH2 into AMN-X (like AMN-150) and AAMN-X (AAMN- 150) inhibited their photocatalytic efficiencies, which was assigned to the declined separation of photogenerated electrons and hole pairs [20]. The BET specific surface area (SSA) of Ag/Ag3PO4 is determined as 12.1 m2/g. While, the BET SSA of AAMN-120 is measured as 478.95 m2/g (Fig. S9 in Supporting information) due to the large SSA up to 1000 m2/g of MIL-125-NH2 [15, 21]. The higher specific surface area of AAMN-120 can provide enough active sites to enhance the photocatalytic performance. Therefore, AAMN-120 was selected as the most suitable photocatalyst for the successive experiments.
|
Download:
|
| Fig. 2. (a) The adsorption and photocatalytic performance and (b) the photocatalytic reduction rates (k values) of samples prepared toward Cr(Ⅵ); (c) AQE of Cr(Ⅵ) reduction over AAMN-120 at various monochromatic light; (d) Photocatalytic Cr(Ⅵ) reduction efficiencies at different pH values over AAMN-120. Reaction condition: initial Cr(Ⅵ) concentration is 10 mg/L, the AAMN-120 dosage is 20 mg, volume is 80 mL, pH 2.0. | |
To determine the apparent quantum efficiency (AQE) during the Cr(Ⅵ) reduction, several optical filters (FWHM = 15 nm) were adopted to yield incident light with different wavelengths (Fig. 2c, Table S1 in Supporting information). The results revealed that the AQE curve was consistent with the UV-vis DRS plot, clarifying that the Cr(Ⅵ) reduction was driven by photo-induced process.
As illustrated in Fig. 2d, the highest photocatalytic Cr(Ⅵ) sequestration efficiency of occurred at pH 2.0 (91.0% in 50 min and 100.0% in 70min) (Table S2 in Supporting information). Under acid condition, sufficient H+ canpromote the transformation of Cr(Ⅵ) to Cr(Ⅲ) (following Eqs. (1 and 2) [8]. With the increase of pH, the zeta potential (Fig. S10 in Supporting information) of AAMN-120 became more negative, which could electronically repel with Cr2O72- and CrO42-. As the pH further increased to ca. 6.0, the Cr(Ⅵ) sequestration followed Eq. 3 [20], inwhich the formed Cr (OH)3 precipitates on the surface of AAMN-120 will cover the active sites to inhibit the photocatalytic efficiency.
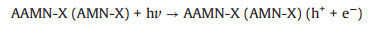
|
(1) |
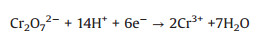
|
(2) |
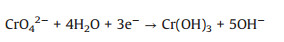
|
(3) |
To check the influence of different hole scavengers toward photocatalytic Cr(Ⅵ) sequestration, tartaric acid, oxalic acid and citric acid were introduced into the Cr(Ⅵ) reduction reaction system (pH 2.0). The photocatalytic reaction rates before 50 min followed the order of tartaric acid > citric acid > oxalic acid (Fig. S11a in Supporting information), which is consistent with the number of α-hydroxyl carboxylate groups in small organic acids [22]. Organic matter in lake water can consume holes and accelerate the separation of electrons holes pairs, thus accelerating Cr(Ⅵ) sequestration (Fig. S11b in Supporting information). It was worth noting that inorganic ions in tap water (Table S3 in Supporting information) exerted no obvious inhibition on the Cr(Ⅵ) sequestration ability.
The active species capture experiment can provide some solid information to understand the intrinsic reaction of photocatalytic Cr(Ⅵ) sequestration (Fig. S11c in Supporting information). The addition of EDTA-2Na enhanced the photocatalytic Cr(Ⅵ) reduction activity as it can capture the formed holes. The Cr(Ⅵ) sequestration efficiency was greatly suppressed by pumping N2 into the reaction system because the introduction of N2 gas can inhibit the formation of O2·- radicals. It was believed that both O2·- and photo-induced e- controlled the Cr(Ⅵ) reduction process. The electron spin resonance (ESR) measurements results further confirmed that O2·- radicals were produced in the photocatalytic process (Fig. S11d in Supporting information).
The recyclability and stability of the AAMN-120 photocatalyst was also investigated (Fig. 3a). After five cycles, AAMN-120 maintained excellent photocatalytic activity, and there was no change in PXRD (Fig. 3b), FTIR spectra (Fig. S12a in Supporting information) and SEM (Fig. S12b in Supporting information). And the XPS after photocatalytic reaction further confirmed the integrity of the surface chemical state of AAMN-120 (Fig. S13 in Supporting information). Further and detailed analyese of PXRD, FTIR, SEM, XPS spectra were listed in Supporting information. Being compared with the counterpart photocatalysts, the photocatalytic activity of AAMN-120 is higher for Cr(Ⅵ) reduction under the identical reaction conditions (Table S4 in Supporting information).
|
Download:
|
| Fig. 3. (a) The reusability of AAMN-120 toward Cr(Ⅵ) under visible light. (b) Comparison of PXRD before and after five cycles of photocatalytic Cr(Ⅵ) over AAMN-120. | |
The lower PL peak of AAMN-120 (Fig. S14a in Supporting information) informed us that the recombination of photogenerated e- and h+ was effectively inhibited [23], which was confirmed by the transient current response results (Fig. S15 in Supporting information). The EIS (Fig. S14b in Supporting information) clearly expressed that AAMN-120 had the smallest Nyquist radius, suggesting that the highest charges separation rate and the fastest charge transfer was achieved in AAMN-120 [24].
The EFB of MIL-125-NH2 was measured to be ca. -0.58 eV vs. the Ag/AgCl electrode at pH 2 (Fig. S14c in Supporting information). Therefore, HOMO of MIL-125-NH2 was calculated as 2.29 eV vs. NHE at pH 2.0. The valence band (VB) of Ag3PO4 was determined to be 2.53 eV with the aid of XPS measurement (Fig. S14d in Supporting information). Combined with Eg value of Ag3PO4, the conduction band (CB) of Ag3PO4 should be 0.2 eV, which is consistent with that in other literatures [7, 16, 25]. In order to further determine the transfer path of photogenerated electrons, the photo-deposition of Pt was conducted over AAMN-120.
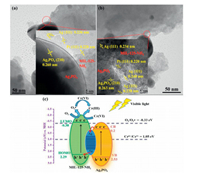
It can be seen from Figs. 4a and b that Ag NPs not only cover MIL-125-NH2 and Ag3PO4, but also exist at their junction, which provides an efficient medium for the possible charge transfer between them. It can be observed that the Pt element after photo-deposition mainly adhere to the MIL-125-NH2, indicating that there were abundant electrons aggregation on the MIL-125-NH2 [19, 26].
|
Download:
|
| Fig. 4. (a) and (b) TEM image of Pt-loaded AAMN-120 (inserted figure is HRTEM of corresponding TEM area). (c) Schematic illustration and the proposed mechanism of photocatalytic Cr(Ⅵ) cleanup over AAMN-120 under visible light irradiation. | |
On the basis of the above discussion and analyses, the possible Z-scheme mechanism of Cr(Ⅵ) cleanup was proposed (Fig. 4c). When the AAMN-120 composite was irradiated by visible light, both MIL-125-NH2 and Ag3PO4 can be excited to produce photogenerated electrons and holes. The photo-induced e- over CB of Ag3PO4 could easily migrate to Ag0 NPs (electrons transfer Ⅰ: Ag3PO4 CB → Ag0) through the Schottky barrier owing to that the CB potential of Ag3PO4 was more negative than the Fermi level of metal Ag0 (2.3 eV) [27]. The Fermi level of Ag0 NPs was more positive than the HOMO of MIL-125-NH2. Therefore, the holes accumulated over the HOMO of MIL-125-NH2 could also easily flow to Ag0 NPs (electrons transfer Ⅱ: Ag0 → MIL-125-NH2 HOMO), which was faster than the recombination of the charge carriers over the HOMO and LUMO of MIL-125-NH2 [17]. Finally, the Z-scheme electron transfer occurs in the Ag/Ag3PO4/MIL-125-NH2 systems, in which Ag0 NPs act as the electrons transfer medium to promote the separation of photo-induced charge carriers. The photo-induced electrons over MIL-125-NH2 can directly involve in the reduction of Cr(Ⅵ), which was consistent with the experimental results of introducing nitrogen into the system (Fig. S11c). Meanwhile, the e- on the LUMO of MIL-125-NH2 were captured by O2 continuously to form O2·- to further reduce Cr(Ⅵ) (E(O2/O2·-) = -0.33 eV vs. NHE). Therefore, the effective transfer of photogenerated carriers in the Z-Scheme Ag/Ag3PO4/MIL-125-NH2 heterojunction led to the enhanced photocatalytic Cr(Ⅵ) cleanup.
In summary, we have developed a highly efficient and stable photocatalyst (AAMN-X) for the reduction of hexavalent chromium under visible light, in which AAMN-120 composite possessed excellent properties toward Cr(Ⅵ) sequestration at pH 2.0. It was found that some small organic acids and dissolved organic matters in lake water can accelerate the reduction of Cr(Ⅵ), while the inorganic ions in tap water can inhibit the photocatalytic ability. The improvement of photocatalytic activity was assigned to the enhancement of photogenerated electrons and holes transfer with the aid of the Z-scheme heterojunction. The ESR, active species capture experiments, and Pt deposition experiments proved that both O2·- and photogenerated e- controlled the Cr(Ⅵ) reduction. It was an effective and controllable strategy to prepare the Ag0/Ag-based semiconductors composites via the in-situ self-sacrifice reaction of Ag+ into Ag0 upon the irradiation of visible light, which can be used to modify some metal-organic frameworks (MOFs) to accomplish boosted photocatalytic performance for environment remediation and ecological sustainability.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by Beijing Natural Science Foundation (No. 8202016) and Beijing Talent Project (No. 2019A22).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.02.048.
| [1] |
C.C. Wang, X.D. Du, J. Li, et al., Appl. Catal. B: Environ. 193 (2016) 198-216. DOI:10.1016/j.apcatb.2016.04.030 |
| [2] |
T. Zhang, W. Lin, Chem. Soc. Rev. 43 (2014) 5982-5993. DOI:10.1039/C4CS00103F |
| [3] |
R.J. Kuppler, D.J. Timmons, Q.R. Fang, et al., Coord. Chem. Rev. 253 (2009) 3042-3066. DOI:10.1016/j.ccr.2009.05.019 |
| [4] |
Y.X. Li, H. Fu, P. Wang, et al., Environ. Pollut. 256 (2020) 113417. DOI:10.1016/j.envpol.2019.113417 |
| [5] |
P. Zhou, J. Yu, M. Jaroniec, Adv. Mater. 26 (2014) 4920-4935. DOI:10.1002/adma.201400288 |
| [6] |
C. Cui, Y. Wang, D. Liang, et al., Appl. Catal. B: Environ. 158- 159 (2014) 150-160. |
| [7] |
F.A. Sofi, K. Majid, O. Mehraj, J. Alloys. Compd. 737 (2018) 798-808. DOI:10.1016/j.jallcom.2017.12.141 |
| [8] |
X.H. Yi, S.Q. Ma, X.D. Du, et al., Chem. Eng. J. 375 (2019) 121944. DOI:10.1016/j.cej.2019.121944 |
| [9] |
D.D. Chen, X.H. Yi, C. Zhao, et al., Chemosphere 245 (2020) 125659. DOI:10.1016/j.chemosphere.2019.125659 |
| [10] |
X. Wang, W. Liu, H. Fu, et al., Environ. Pollut. 249 (2019) 502-511. DOI:10.1016/j.envpol.2019.03.096 |
| [11] |
H. Wang, X. Yuan, Y. Wu, et al., J. Hazard. Mater. 286 (2015) 187-194. DOI:10.1016/j.jhazmat.2014.11.039 |
| [12] |
S. Nayak, K.M. Parida, ACS Omega 3 (2018) 7324-7343. DOI:10.1021/acsomega.8b00847 |
| [13] |
D. Sun, W. Liu, Y. Fu, et al., Chem. Eur. J. 20 (2014) 4780-4788. DOI:10.1002/chem.201304067 |
| [14] |
G. Fan, X. Zheng, J. Luo, et al., Chem. Eng. J. 351 (2018) 782-790. DOI:10.1016/j.cej.2018.06.119 |
| [15] |
B. Kim, Y.R. Lee, H.Y. Kim, W.S. Ahn, Polyhedron 154 (2018) 343-349. DOI:10.1016/j.poly.2018.08.010 |
| [16] |
J. Lu, Y. Wang, F. Liu, L. Zhang, S. Chai, Appl. Surf. Sci. 393 (2017) 180-190. DOI:10.1016/j.apsusc.2016.10.003 |
| [17] |
L. Ye, J. Liu, C. Gong, et al., ACS Catal. 2 (2012) 1677-1683. DOI:10.1021/cs300213m |
| [18] |
J. Qiu, M. Li, H. Wang, J. Yao, Chemosphere 242 (2020) 125197. DOI:10.1016/j.chemosphere.2019.125197 |
| [19] |
W. Zhao, T. Ding, Y. Wang, et al., Chin. J. Catal. 40 (2019) 1187-1197. DOI:10.1016/S1872-2067(19)63377-2 |
| [20] |
Y.C. Zhou, X.Y. Xu, P. Wang, et al., Chin. J. Catal. 40 (2019) 1912-1923. DOI:10.1016/S1872-2067(19)63433-9 |
| [21] |
S.N. Kim, J. Kim, H.Y. Kim, H.Y. Cho, W.S. Ahn, Catal. Today 204 (2013) 85-93. DOI:10.1016/j.cattod.2012.08.014 |
| [22] |
X.H. Yi, F.X. Wang, X.D. Du, P. Wang, C.C. Wang, Appl. Organomet. Chem. 33 (2019) e4621. DOI:10.1002/aoc.4621 |
| [23] |
J. Chen, J. Zhan, Y. Zhang, Y. Tang, Chin. Chem. Lett. 30 (2019) 735-738. DOI:10.1016/j.cclet.2018.08.020 |
| [24] |
Z. Cai, X. Hao, X. Sun, et al., Water Res. 162 (2019) 369-382. DOI:10.1016/j.watres.2019.06.017 |
| [25] |
F.A. Sofi, K. Majid, Mater. Chem. Front. 2 (2018) 942-951. DOI:10.1039/C8QM00051D |
| [26] |
W. Jiang, X. Zong, L. An, et al., ACS Catal. 8 (2018) 2209-2217. DOI:10.1021/acscatal.7b04323 |
| [27] |
C. Liang, C.G. Niu, H. Guo, et al., Catal. Sci. Technol. 8 (2018) 1161-1175. DOI:10.1039/C7CY02190A |
 2020, Vol. 31
2020, Vol. 31 

