b State Key Laboratory of Hydraulics and Mountain River Engineering, College of Architecture and Environment, Sichuan University, Chengdu 610065, China
Antibiotic resistance genes (ARGs), which were typical emerging contaminants, have drawn considerable attention due to their negative effectiveness in pathogenic bacterial infections therapy during antibiotics treatments [1]. According to reports of Centers for Disease Control and Prevention (2013), more than 2, 000, 000 people in the United States were affected by antibiotic-resistant infection annually, resulting in 23, 000 deaths every year [2, 3]. Similarly, approximately 25, 000 deaths, in the EU, were caused by antibiotic resistance bacteria (ARB) in 2009 [4]. Ten million deaths caused by ARGs are estimated in 2050 throughout the world [5]. Although new antibiotics are continuously explored to kill pathogenic resistant bacteria, a decline in the accompanied therapeutic effects was recently reported. For instance, the World Health Organization (WHO) pronounced that treatment failure of third generation cephalosporin antibiotics to gonorrhea, has been confirmed in at least 10 countries (Australia, Austria, Canada, France, Japan, Norway, Slovenia, South Africa, Sweden and the United Kingdom of Great Britain and Northern Ireland) [6]. If no action is taken to cope with the ARGs crisis, undoubtedly, we will return to an era without antibiotics. Regarding the serious threat of ARGs to public health, the WHO defined ARGs as one of the top three critical challenges of the 21st century [7].
Antibiotic resistances occur, either by intrinsic resistance or acquisitions that confer resistance via mutations and horizontal gene transfer (HGT). The latter one has been always regarded as the most important factor for the current multiple resistance pandemic. The HGT mechanism of ARGs among bacteria can be summarized as: (1) Conjugation via cell-to-cell contact due to the action of mobile genetic elements (MGEs), such as integrons, transposons and plasmids [8]; (2) transduction (delivery of genetic material through phage); (3) transformation via extracellular DNA (eDNA) uptaking. Recently, considerable extracellular ARGs (eARGs), which are secreted by living antibiotic resistance bacteria (ARB) or originated from the lysis of dead ARB have been observed in the natural environment [9]. The recent publication of Dong et al. (2019) reported that extracellular ARGs, which were extracted from hospital sludge and urban lake sediment, were higher than intracellular ARGs [10]. This finding demonstrates that ARGs can transcend their bacterial host and exhibited characteristics of easy propagation [11]. Another unique feature of ARGs is that many triggers (such as heavy metals, organic matter etc.), promote the dissemination of ARGs [12, 13]. The propagation of ARGs contributed to a pollution potential that is more serious than the corresponding antibiotics and ARB. To clarify the occurrence, mitigation and negative effects of ARGs on human beings and environments, topics such as (1) detection and quantitative analysis of ARGs, (2) molecular mechanisms of various known resistance genes, (3) migration and transformation of ARGs in complex matrices, and (4) methods for the control of ARGs have been extensively investigated. Wastewater treatment plants (WWTPs), which receive ARGs from various sources via sewage drainage systems, are reservoirs for ARGs and have an important role in ARGs conservation, removal and propagation [14]. The higher is the abundance of ARGs in the influent of WWTPs, the greater are the opportunities for the transference of their genes [15-17]. However, traditional WWTPs were designed for the removal of traditional pollutants, such as nitrogen, suspended solids, total phosphorus, organics and total coliforms [18], and seldom focused on antibiotic resistance. Thus, comprehensively understanding the sources of ARGs within the influent of WWTPs, and the performance of existing terminal treatment approaches for ARGs removal in WWTPs are urgent. Undoubtedly, relevant research was meaningful for formulating better wastewater management strategies for reducing the mitigation risks of ARGs. In this paper, first, we analyze the research hotspots of ARGs. Our analysis revealed increasing concerns regarding ARGs in WWTPs. We focused our research on this subject and we summarized the potential sources of ARGs in WWTPs. Second, the selective pressure of different contaminants in WWTPs on ARGs is evaluated using the correlation coefficients as indexes. Third, the conventional and recently springing-up treatment strategies for ARGs removal is emphasized analyzed. Last, future research directions for the control of ARGs controlling are presented.
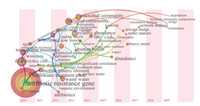
2. Analysis of relevant research hotspotsTo comprehensively understand the research hotpots of ARGs in recent years, a temporal visualization map, which is based on the related publications of ARGs from 2010-2019 (SCI-expanded, SSCI, A&HCI SSCI), was obtained. The results are shown in Fig. 1 (using Citespace software). The frequency of the keywords reflected the research hotspot [19, 20]. As illustrated in Table S1 (Supporting information), "waste water", "wastewater treatment plant", "removal" and "degradation" were 173, 114, 80 and 21 times, respectively, suggesting that the profile of ARGs in wastewater and subsequent treatment approaches have received more attention.
|
Download:
|
| Fig. 1. Evolutional tendency of related research on ARGs from 2010 to 2019. | |
The burst keywords refer to some hotspots that appear more frequently over a period of time in a certain field. The biggest burst strength indicates the latest research frontier [20]. The widespread utilization of antibiotics and the serious antibiotic resistance crisis in China [21] may explain the steady grow studying of ARGs, which can be proven by the burst strength of 15.67 for keyword "China". "Relative abundance" has been a hotspot since 2013 (frequency of 186), and thekeywordsof"heavymetal"and"significant correlation" became the frontiers, with a burst strength of 3.29 and 1.95, respectively. These finding revealed that the variationin the research focuses on from simple quantifying of ARGs to ARGs propagation.
Although research has engaged tremendous enthusiasm in ARGs research during the past decades, the noticeable prevalence of ARGs in WWTPs remains grim throughout the world. Thus, "sewage treatment plant" remains at the forefront of a recent publication (burst strength = 3.02), and the related treatment methods such as "activated sludge" (2.69), have also attracted the attention of researchers.
3. Potential sources of ARGs in WWTPs influentGenerally, synthetic antibiotics, which have been widely utilized in disease therapies, cannot be completely metabolized by organisms and as much as 60%–85% of antibiotics are directly discharged into drainage systems or aquatic environments via human/animal excrement [22, 23]. Moreover, low doses of antibiotics are usually added to the feed of animals for the prevention of diseases. Thus, long periods of the utilization of antibiotics may cause the proliferation of ARGs in wastewater. We summarised the abundance of ARGs in different sources for WWTP influent in Table 1 [24-36]. Since the transportation of wastewater in the sewage drainage always resulted an insignificant reduction of ARGs [37], consequently, WWTPs serve not only as "tanks" for resistant organisms and antimicrobials from an extensive variety of sources (i.e., livestock, hospitals, industries, and households) but also potential breeding sites for ARGs dissemination. The discharging of ARGs to natural waterbodies via the WWTPs effluent, which was the main path for the propagation of antibiotic resistance to indigenous bacteria (Fig. S1 in Supporting information) [38]. Thus, understanding the source of ARGs within the influent of WWTPs is fundamental to listing the critical targets for the mitigation and control of ARGs and further formulation of control strategies.
|
|
Table 1 Abundance of ARGs in different sources for WWTP influent. |
The subclinical dosage of antibiotics has been added to animal feed to control animals' epidemics and enhance the growth of animals in livestock and poultry industries [39]. A recent publication reported that the consumption of antibiotics in livestock and poultry industries in China reached 84, 240 tons in 2013, which accounts for as much as 52% of the bulk antibiotics productivity [40]. Routine administration of antibiotics to animals at inappropriately low-doses (ng/L–μg/L) caused the prevalence of ARB and ARGs in the excrements of pigs, cows, ducks and chicken [41, 42]. Moreover, the water flushing of animal faeces/urine further deteriorated the propagation of ARGs in livestock breeding wastewater [43]. The investigation of Chen et al. discovered 22 ARGs in the wastewater of 12 livestock farms (four pig farms, four cattle farms, and four chicken farms), in which the most abundant genes (in copies/16S rRNA gene copies) were sul1 (3.84 × 101), sul2 (1.62 × 101) and tetM (2.33 × 101) [24]. Correspondingly, Ben et al. found that tetC and tetO were the most abundant of existing ARGs in swine wastewater (averaged 7.3 × 10-3 and 1.7 × 10-1 copies/16S rRNA gene copies) via the investigation of 7 target ARGs in 9 swine feedlots [25]. Moreover, Sui et al. observed that the gene copies of ARGs in two typical swine farms reached to 1E + 08 copies/mL (same multitude) regardless of the locations of feedlots [27]. Abundant sources of the frequently detected ARGs in the influent of WWTPs are summarized; the results are listed in Table 1. Specifically, tetracyclines, sulphonamides and macrolidesrelated ARGs are among the most detectable ARGs in livestock and poultry breeding wastewater, due to their widespread utilization in livestock farms [44-46].
Except for the chemical characteristics of antibiotics, the species, growth phase of animals, and resistance mechanism influenced the distribution and diversity of ARGs in livestock breeding wastewater [47]. According to the survey of Zhang et al., ARGs in piggery wastewater was higher than those obtained from cattle ranch [48]. The frequency of antibiotic administration for cattle of younger ages likely influences the distribution of microbiome and resistome in ways that reflect higher ARGs abundance [49, 50]. For different ARGs, the abundance of ribosomal protection protein genes (tetQ, tetM, tetW, and tetO) was higher, in a wastewater lagoon, than majority of the efflux pump genes (tetA, tetB, tetC, and tetL) and enzymatic modification gene (tetX) [51]. In addition, antibiotic use strategy and seasonal condition changes also brought ARGs fluctuating [52, 53]. Peak et al. indicated that the concentration of ARGs in autumn was approximately 10-100 times higher than that in summer in the wastewater of cattle feedlots [54]. Similarly, Sui et al. revealed that the abundance of ARGs in raw swine wastewater in winter was 0.31–3.52 logs higher than that in summer [55]. Low temperature interrupt the ARB's survival but prevent antibiotics' degradation, might be the main reasons for abovementioned observation [56, 57].
Tremendous on-farm waste lagoons were built for most concentrated animal feeding operations (CAFOs) to store wastewater flushed from barns. Despite the majority of the collected wastewater was treated with a septic tank or biogas digester, the uncompleted reduction of those antibiotics by these approaches led to a significant ARGs emission [51]. For example, the relative abundances of the ARGs of tetC, tetG, sul1, and sul2 even increased by 0.74–3.90 logs after septic tank or biogas digester treatment [25]. In addition, scattered livestock, which has been subjected to effortless wastewater disposal technology, always discharged more ARGs into aquatic environments and aggravated pollution. Consequentially, the emission of these livestock and poultryrelated ARGs into sewage drainage caused the operational stress of WWTPs. Therefore, how to strengthen the on-farm treatment of ARGs should be addressed to eliminate the prevalence of ARGs in WWTPs. In addition, green and food-grade disinfectants should be widely explored, for substituting antibiotics and avoiding ARGs prevalence, to reduce ARGs discharging into WWTPs from livestock wastewater. Meanwhile, biodegradable material such as wood chips were also applied to absorb excrement to minimize wastewater volume, to alleviate the transferring of ARGs in water flushing of the animal urine and faeces. Moreover, management strategies could shift with the seasons, species and growth phase and prioritize wastewater with "higher risk" to solve burning issue.
3.2. ARGs from antibiotic manufactures, hospitals and householdsAs previously mentioned, the rapid spread in environmental matrices was closely related to the gross utilization of antibiotics in humans, animals and agriculture [58, 59], and a high level of antibiotics exerts a fairly high selective pressure on the maintenance of ARGs [60, 61]. Thus, wastewater from antibiotic manufactures, hospitals and households have been highly concerning due to their higher antibiotic residues than any other wastewaters [62, 63]. For example, the high levels of oxytetracycline (OTC) antibiotics (0.36–12.36 mg/L) in OTC production wastewater produced a higher concentration of tetracycline resistance genes (tet genes) in wastewater (1–4 orders magnitude higher than sewage wastewater), with the highest abundance of 1.8 × 100 copies/16S rRNA copies for tetQ [28]. The emission of the corresponding ARGs in wastewater treatment systems negatively deteriorated the bulk quality of the WWTPs effluent [28, 64]. Consequently, the maximum concentration of the detectable ARGs in the effluent of pharmaceutical wastewater treatment plants (PWWTPs), reached 10-2 to 10-1 copies/16S rRNA copies (sul1, sul2, qacE, qacE△1, tetA) in the Czech Republic [29] and reached 2.36 × 107 copies/mL (sul, tet, OXA, and erm) in the PWWTP effluent in northern China [30]. In addition, the concentration of these ARGs, which originated from chromosomal mutations, would decline significantly with the effective biodegradation of antibiotics during the operation of PWWTPs [18]. The recent works of Guo et al. [18] and Meng et al. [65] revealed that the vertical gene transfer in antibiotic manufactures wastewater was the main driver for the propagation of ARGs rather than horizontal transference. This finding suggests that enhancing the removal of antibiotics may be a suitable strategy for controlling the dissemination of ARGs for antibiotic manufactures.
Comparatively, antibiotics in the wastewater of the partial manufacturing plants are staggering, concentration even close to or above therapeutic levels (mg/L range), constituting strong drivers of resistance [38]. Antibiotic residuals may negatively suppress the biomass activity during biological treatments, increasing the operational difficulties. Besides, typical production processes of antibiotics mainly include filtration, refinement, extraction, crystallization, centrifuge, drying, and packaging [66], and process control strategy might be the first choice if the terminal treatment is inefficient. Moreover, rational selection of antibiotic production method is also beneficial to control ARGs pollutions. For instance ARGs concentration in the wastewater of the pharmaceutical companies, which using fermentation techniques, were much higher than those semi-synthetic, and synthetic processes [18]. Furthermore, more strict emissions standards for ARGs and antibiotics discharging were necessary for pharmaceutical industries.
Similar to pharmaceutical wastewater, wastewater from hospitals exhibited a relatively high concentration of antibiotics and ARGs due to the incomplete metabolism of patients [67]. Li et al. observed a noteworthy distribution of tetracycline resistance genes in five hospital samples in China, which was the highest for tetQ (2.80–7.47 × 10-1 copies/16S rRNA), followed by tetO, tetW and tetM [32] and related to its extensive application in ointments [68]. Moreover, a significant variety of ARGs have been detected in hospital wastewater. According to the recent investigation of Wang et al., as much as 131–139 unique ARGs of the 178 targeted genes were detected in three selected typical hospital wastewaters, with a concentration that ranged from 1.88 ± 0 copies/mL (erm A) to (8.34 ±1.23) × 107 copies/mL (tetO) [69]. The concentration of tetracyclines and sulphonamides-related ARGs in the influent of WWTPs exhibited a positive correlation to that of the ARGs in the corresponding hospital wastewater [70]. This finding implies that discharging medical wastewater via sewerage system contributed to the dissemination of ARGs to WWTPs. Conversely, other researchers stated that hospitals were not the predominant sources of the ARGs in sewage WWTPs according to a comparison of the distribution of the ARGs in the influent of WWTPs with and without hospital sewage collection [71, 72]. This finding was ascribed to the relatively low contribution of hospital sewage (an estimated 0.8%–1%) to the bulk volume of WWTP influent [35, 73].
Compared to other wastewater, hospital sewage contained relatively high levels (39.4%± 2.5% of the total microbiota) of anaerobic bacteria partially originate from human gut [74], and would be easily converted to antibiotic resistant bacteria with selective pressure exerted by antibiotics [75]. Therefore, some special on-site sterilized methods should be highly concerned in hospital wastewater treatment, for eliminating anaerobic bacteria and controlling ARGs spreading.
Excessive usage of antibiotics in households was another main contributor of ARGs in WWTPs. A maximum antibiotics consumption by human beings of 75% in Germany was related to the household utilization, this ratio was approximately 80%–95% for Europe [76], 70% for the U. K. [77], and 75% for the U. S. A. [78, 79]. Theoretically, the ARGs contribution to the WWTP influent is higher than that to hospital wastewater. This finding can be shown by the recent work of Li et al., who discovered that hospital wastewater generally exhibited a lower total ARGs abundance than the samples collected from residential areas (4.62 × 107-5.70 × 1011 copies/mL vs. 2.28 × 1011-3.41 × 1011 copies/mL) [34]. Similarly, Aali et al. reported that tetW in household wastewaters ranged from 3.87 × 105 to 6.23 × 1013 copies/mL, which was considerably higher than that of hospital wastewater (1.13 × 105-7.6 × 1010 copies/mL) [35]. More seriously, utilization of antibiotic in certain areas was always unregulated [80]. Thus, long durations, excessive doses and unscientific utilization of antibiotics in residential areas should be addressed to control ARGs pollution [81]. Thus, we should pay more attention to restricting the abusing utilization of antibiotics to alleviate their selection pressure on ARGs propagation. Besides, the random discharge of domestic sewage in rural areas, as compared to urban domestic sewage, could not be efficiently collected and thus with great concerning. Therefore, it is practically urgent to focus on treatment of the domestic sewage in rural areas.
3.3. ARGs in initial stormwater runoffA series of point and non-point sources, including road, defective septic systems, fertilized manure, and agricultural runoff, also contributed to ARGs pollution in stormwater runoff [36], evidenced by the higher concentration of ARGs in the initial runoff than that in urban wastewater [82]. Extensive utilization of the combined drainage systems caused the tremendous discharge of ARGs into WWTPs via initial stormwater runoff collection [83]. Moreover, rainfall, which increases the WWTP hydraulic load significantly reduced the removal of ARGs [84]. Numerous studies have proven that rainfall increased the loadings of ARB and ARGs in surface water but seldom focused on clarifying the interaction of the stormwater ARGs and the water quality of WWTP influents [85, 86]. For example, Baral et al. discovered that the overflow storm in rain conditions contributed as much as 82%–88% of the ARGs within the urban stream, in which the runoff from streets was the predominant contributor (accounted for 92%–96% of ARGs) [87]. Similarly, Garner et al. [36] stated that the five ARGs (sul1, sul2, tetO, tetW, ermF) in an inland stream were higher in storm loading conditions, especially for sul2 and tetW. These results proved the existence of resistance gene in rainwater and warned us of the need to pay more attention to the influence of rainwater on the influent of WWTP.
Currently, the dissemination of ARGs in streams/WWTPs and possible influencing factors comprised a brand-new research field. Research revealed the approach of broadcast caused a more significant emission of erm genes in runoff than that of incorporation and injection [88]. Moreover, the complex chemical components of stormwater, such as heavy metals, natural organic matters, antibiotics etc., would enhance the selection and amplification of ARGs [89] and subsequently increase the transference risks of ARGs from resistant native aquatic bacteria to pathogenic bacteria [90]. Bacteria can easily survive on soil and dust particles, hence the decline in the solids washed from impervious surfaces may partially reduce the loading of ARGs in stormwater runoff [91]. It arouses us to focus on initial stormwater runoff volume reduction to relieve stress of WWTPs. Stormwater management, such as low impact development (LID) or sustainable urban drainage systems (SUDS) may serve as useful approaches, especially the application of those pervious surfaces, soil infiltration, sunken green and etc.
By far, majority of the works are focused on the clarifying of the potential independent sources of ARGs in WWTPs influent. The contributors of ARGs in the influent of WWTPs and the correlations between different sources and the prevalence of ARGs in WWTP are seldom reported. Thus, more data are required to obtain greater insights into the source contribution status and risk ranking of different sources in ARGs.
4. Stressors of ARGs propagation during WWTPs operationIn addition to ARGs, wastewaters from livestock/poultry breeding, medical facilities, pharmaceutical industries, hospitals and households, as well as stormwater runoff also contained tremendous pollutants of surfactants, antimicrobials, heavy metals and disinfectants (concentration ranged from ng/L to μg/L) [92-94]. The discharges of these pollutants into municipal drainage systems [95], and subsequent into WWTPs influent may place selective pressure on ARGs propagation [96]. Thus, the analysis of the previously mentioned stressors may clarify the propagation mechanism of ARGs in WWTPs. Potential correlation between the typical pollutants and ARGs distributions, which always occurs via bivariate and Pearson correlation analysis, are compared and summarized in Table 2 [13, 97-106].
|
|
Table 2 Potential affected factors and the corresponding correlation of those factors on the ARG propagation. |
Antibiotics have long been recognized as a driving force for ARGs propagation [107, 108], either by exerting selective pressure directly, or by stimulating the transfer of mobile elements which are responsible for ARGs dissemination indirectly [109]. Theoretically, the higher is the emission of incompletely metabolized antibiotics into an aquatic environment, the greater is the generation of ARGs [100, 110]. A significant correlation (R2 = 0.97, P < 0.05) was also observed for sulI genes and the sulphonamides concentration in conventional WWTP effluent [97]. Similarly, Smith et al. revealed that tetO, tetQ and tetW in water samples, which contained a higher tetracycline concentration (> 1.95 μg/L), was 4.0–8.3 fold higher than those with a lower tetracycline concentration (< 1.95 μg/L) [111]. Conversely, Gao et al. observed that the abundance of tetO and tetW did not show a significant correlation with corresponding antibiotics at a low-level tetracyclines (oxytetracycline, chlortetracycline, doxycycline and tetracycline) concentration of 1.1 μg/L [112]. Analogously, no significant correlation was observed, between tetR and tetracyclines (concentration < 7.74 μg/L) in a recent publication of Zhang et al. [48]. Beside antibiotics themselves, intermediates are frequently reported as inducements of antibiotic resistance during biological wastewater treatments [113, 114]. Since the assessment of the bioavailable concentrations of antibiotics in different bacterial species in wastewater is quite difficult, thus always caused an over- or underestimation of the selection pressure of AGRs.
In many cases, antibiotics induce ARGs propagation by promoting the generation of mobile genetic elements (integron, plasmid, transposon, bacteriophage and so on), which carry one or more gene cassettes that encode with multiple antibiotics resistance [115, 116]. Study showed that 100 ppb sulfamethoxazole promoted intl1 generation significantly, resulting in sul1 consistently becoming more abundant [36, 117]. In addition, selective pressure exerted by antibiotics on mobile genetic elements trigger co-selection. Dang found cat genes (chloramphenicol-resistance gene) propagation was most likely the result of cross-selection by oxytetracycline [118]. Avoparcin, which is a glycopeptide antibiotic that is utilized as growth-promoter in poultry, was banned for its association with the productivity of vancomycin-resistant enterococci [119].
From above, we can conclude that following characteristics were responsible for antibiotics in inducing the generation of resistance genes: (1) Threshold value. Concentration of antibiotics had a substantial role during ARGs propagation, however, correlation between antibiotics and their corresponding resistance genes was not usually positive. The selective pressure on ARGs was closely related to their threshold value [48]. (2) Metabolites and intermediates still have certain biological activity. Unlike other pollutants, antibiotics, as well as their metabolites, intermediates, or precursors in wastewater can significantly affect the bacteriostatic activity [120-124], which also affects the microbial community, and promotes generation and spread of bacterial resistance. Therefore, bioavailable concentrations of antibiotics should be emphasized and analysed for efficient control of the spread of ARGs. (3) Co-selection. Selective pressure of antibiotics on mobile genetic elements may bring co-selection, thus development of resistance to corresponding antibiotic is accompanied by resistance to another agent. (4) Inactivation of other functional bacteria. Antibiotics within WWTPs influent would decline the bulk removal efficiency of ammonia nitrogen, cause variation in the community structures of functional bacteria, and negatively affect the performance of WWTPs [125]. Thus, the efficient removal of the right groups of active antibiotics from WWTPs influent would not only guarantee the alleviation of ARGs selection pressures but also benefit the removal of traditional pollutants [126].
4.2. Heavy metalsHeavy metals were another essential factor that influenced the proliferation of ARGs [127], which might explain why the decrease in antibiotics concentration could not effectively prevent the spread of ARGs. Under heavy metals stress, bacteria acquire antibiotic resistance through (1) co-resistance, i.e., antibiotic and heavy-metal resistance genes bind onto a same genetic element such as plasmid, integron and transposon [105, 128]; (2) crossresistance, the same genetic determinant such as efflux pumps responsible for resistance to dissimilar compounds [129]; (3) co-regulation, forming a coordinated response to metal or antibiotic, after a range of transcriptional and translational responses to either stress exposure have be linked [130]. ARGs are continually screened and enriched in bacteria, showing an increase in resistance levels.
With the significant emission of wastewater from small enterprises, local urban runoff, traffic emissions and other anthropogenic-derived sources, non-biodegradable heavy metals were always the most frequently detectable pollutants in WWTPs and have been regarded as representative of the long-term selective pressures of ARGs. For instance, Ma et al. verified that Cu2+ removal efficiencies were relatively lower than chloramphenicol (less than 20%) in a bio-electrochemical system and the proliferation of cmlA were enhanced when Cu2+ concentration increased from 10 mg/L to 50 mg/L [131]. Moreover, Mao et al. revealed a significant correlation with the concentration of heavy metals (Hg, As, Zn, Ni and Pb) and ARGs (sul1, tetB, tetG, tetH, tetX) in two typical WWTPs, even with a low heavy metals concentration condition (in μg/L) [101]. Similarly, Gao et al. discovered that Zn (33-112 μg/L) was significantly correlated with the ARGs of ereA, ereB, mefA, mefE, and ermB (0.823 < R < 0.866, P < 0.05) in urban wastewater [103].
In addition, co-selection of Zn, Cu (in the form of CuSO4) and antibiotics are frequently reported in the livestock and poultry breeding field, because abovementioned two metals are commonly employed as supplements in animal feed production [132, 133]. A noteworthy correlation between ARGs and Zn, Cu have been extensively reported; some of the results are listed and discussed in Table 3 [98, 101, 106, 134-144]. Thus, we can conclude that reducing the utilization of antibiotics or metals during animal feed production and enhancing the removal efficiency of these pollutants via pre- or enhanced treatment units would alleviate the selective pressures of ARGs in WWTPs.
|
|
Table 3 Removal efficiency of ARGs during different processes. |
Theoretically a long-term selection pressure of heavy metals would be observed, since metal ions are recalcitrant and not subject to degradation, especially under the conditions that heavy metals are currently several orders of magnitude higher than antibiotics [145]. Reports have confirmed that the level of antibiotic resistance genes increases with deterioration of heavy metal contamination [146]. Scholars have proposed some methods to reduce or eliminate the toxicity of free metal ions in the animal fodder, via conversion them to an organic form [147]. Hence, such potential mitigating strategies should be further explored.
4.3. Other contaminantsThe existence of organics, surfactants, disinfectants other compounds within wastewater also significantly affected the emergence, proliferation and spread of ARGs [148]. The recent work of Devarajan et al. discovered that the abundance of ARGs within clinical wastewater strongly correlated with the concentration of organics (R = 0.574-0.846, P < 0.05) [13]. Furthermore, Di Cesare et al. reported that the selection of tetA, ermB, and qnrS was related to the total organic carbon (TOC) of the water samples [101]. Guo et al. speculated that COD-related organics provided sufficient nutrition to ARB, which ensured that these organisms grew normally even under high pressure of antibiotics. They also determined that the abundance (copies/mL) of sulI in a sequencing batch reactor increased from 1.39 × 105 to 5.99 × 105 to 5.93 × 105 when the COD increased from 100 mg/L to 300 mg/L and 600 mg/L (10 mg/L SMX concentration) [125]. Thus, the decline of the foodto-microorganisms ratio would restrain the energy supply of the ARB, and subsequently increase the metabolic burden of resistance plasmid replication, was meaningful for ARGs controlling.
Except for traditional organic pollutants, the abundance of ARGs was also well correlated with biomass-related protein and polysaccharides, which could be proven by the high-correlation coefficients between the protein and polysaccharide components and ARGs (R = 0.79 and 0.82 for blaTEM and vanA, respectively) [106]. Surfactants, disinfectants, antiseptics such as quaternary ammonium compounds and triclosan, are frequently added to items as diverse as soaps, lotions, toothpaste, and many commonly used household fabrics and plastics. Exposure of susceptible strain to them selected multidrug-resistant bacteria at high frequencies [149]. Several studies revealed that the exposure of quaternary ammonium compounds (QACs), triclosan, pesticides and herbicides enhanced the proliferation of ARGs [150-152]. These chemical reagents indirectly affect the microbial community structure, thus enhanced the transferring and spreading of resistance genes [153]. Pal et al. indicated that QACs and metals can co-select ARGs when bacteria harbour resistance genes towards both types of compounds [12]. Madan et al. determined that QAC-resistant species (Pseudomonas) enriched after long-term exposure to benzalkonium chlorides (typical QACs) and decreased their susceptibility to penicillin G, tetracycline, and ciprofloxacin [151]. Likewise, Brigitta revealed that tolerance of E. coli to chloramphenicol increased in the presence of dicamba and that to kanamycin increased with the existence of glyphosate [154].
5. Artificial treatment for eliminating ARGs in WWTPsWastewater treatment plants are always regarded as reservoirs of antibiotic genes [34, 155-157]; thus, the removal of these ARGs during the operation of WWTPs is urgent.
Generally, the extensively applied approaches in WWTP approaches, such as biological, physical, chemical and physicalchemical processes, may significantly affect the fate and transformation of ARB and ARGs before they are discharged into an aquatic environment. As shown in Table 3, the removal efficiencies of ARGs during typical treatments, were summarized and discussed to facilitate the understanding of the removal trends of ARB and ARGs in WWTPs.
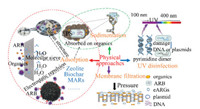
5.1. Physical approachesTypical physical approaches, including adsorption, sedimentation, UV disinfection, and filtration etc. [106], were summarized and discussed here to evaluate the removal behaviours of ARGs in WWTPs (Fig. 2). The porous structure of the natural zeolites, macroporous adsorption resins (MARs) and biochar may have allowed the adsorption of heavy metals to reduce the selective pressure on resistance genes [158]. Biochar and MARs provide the most sites to be filled by sorption of ARB and eARGs, basing on the mechanism of electrostatic repulsion or acting as molecular sieve [159]. Sedimentation tanks were traditionally designed to remove sus-pended substances and activated sludge during the operation of WWTPs. As shown in Fig. 2, eARGs could be efficiently absorbed onto organics and then coprecipitated. Some bacteria, such as Bacteroides which known as host for tetX, can form flocs by excreting extracellular polymeric substances (EPSs), and be easily gravitated via sludge sedimentation [160, 161]. Research confirmed that partial tetA (0.03-0.4 log) and tetB (0.03-0.7 log) can be precipitated via binding onto organics [156]. Mao et al. revealed that most of the screened ARGs (0.95-2.67 logs to the influent) in WWTPs were reduced via sedimentation, and the majority of the ARGs were accomplished within the secondary clarifier unit [101].
|
Download:
|
| Fig. 2. Removal mechanism of ARGs during the operation of the physical approaches. | |
Regarding UV disinfection, the ARGs within wastewater could be directly inactivated via the generation of pyrimidine dimer or an indirect damaging of the DNA structure of microorganisms [162]. UV disinfection efficiency was closely related to the UV intensity and species of the resistance genes. For instance, erythromycinresistant (ereA, ereB, ermA and ermB) and tetracycline resistant genes (tetA, tetB, tetM and tetO) could be effectively inactivated under a 5 mJ/cm2 UV fluence [148], whereas no efficacy was observed for the ARGs of tetQ and tetG, even with UV doses of 30 mJ/cm2 [161]. In general, ARGs required higher UV doses (200–400 mJ/cm2 for 3–4 logs reduction) that are substantially higher than that of ARB (10-20 mJ/cm2 for 4–5 logs reduction) for a complete inactivation [163].
Furthermore, micro- and ultrafiltration were applied by Maria et al. to examine the potential removal trend of ARGs during operations of WWTPs. They discovered that filtration through the 100, 10, and 1 kDa membranes caused an average of 0.9, 3.6, and 4.2 logs reductions of the target ARGs and removal benefited from the existence of colloids [148]. The membrane separation mechanisms of microfiltration (MF) and ultrafiltration (UF) allows those smallsized substances, such as water, organic small molecule and metal ions in wastewater, passed through the pores on the fibers, while majority of the large-sized substances such as ARB are rejected.
5.2. Chemical approachesChemical approaches, including chlorine disinfection, advanced oxidation process (AOPs), coagulation, etc. [164], were extensively applied to the treatment of ARGs-polluted water samples [165]. In the process of chlorine disinfection (Fig. 3), the hypochloric acid could oxidize the cell walls and then react with ARGs.

|
Download:
|
| Fig. 3. Removal mechanism of ARGs during the operation of chemical approaches. | |
Chlorine, the most widely used disinfectant in various countries, is an oxidant that acts by destroying nucleic acids and cell membranes of microorganisms. Theoretically, the higher is the dosage of free chlorine (FC), the higher is the removal trend of ARGs, which could be proven by the noteworthy increase in the removal efficiency of sul1, tetX, tetG, intI1 (< 0.1 log to 1.30–1.49 logs) when the FC dosage increases from 5 mg/L to 30 mg/L [151]. Similarly, Oh et al. discovered that as much as 90% removal of ARGs in the effluent of a biological aerated filter (BAF) process were achieved with a FC dosage of 30 mg/L [166]. Note that the existence of NH3-N within a water sample, competing with free chlorine, will negatively affect the bulk removal efficiency of ARGs. Specifically, the recent work of Zhang et al. discovered that once the dosage of NH3-N increases from 2.56 mg/L to 5 mg/L (30 mg/L FC concentration), the inactivation of ARG will decline from 1.20 to 1.49 logs to 0.63-0.79 logs and further decrease to 0.03-0.10 log when the NH3-N increases from 10 mg/L [167]. This finding might explain why 40% of erythromycin resistance genes (ereA, ereB, ermA, and ermB), as well as 80% of tetracycline resistance genes (tetA, tetB, tetM, and tetO) still existed after chlorination (to 60 mg Cl2 min/L) under the loading of 0.35 kg NH3-N/(m3 d) [150].
As shown in Fig. 3, advanced oxidation process (AOPs), such as Fenton oxidation, photo-Fenton process, TiO2 photocatalysis, and UV/H2O2, were applied in wastewater advanced treatment and reuse [168, 169], during which the DNA double helix structure and plasmids could be destroyed by reactive oxygen species (ROS). The ARGs of sul1, tetX, tetG etc., within the WWTP effluent could be efficiently oxidized during the Fenton oxidation and UV/H2O2 process, which are significantly affected by operational parameters such as initial Fe2+/H2O2 molar ratios, H2O2 concentration, pH, and reaction time (especially for reagent concentrations and pH) [152].
Coagulation, as tertiary treatment, can enhance ARGs removal and reduce risk to aquatic environments. ARB mostly have negative electrical charges while coagulants carry a positive charge. A coagulant neutralizes bacteria and makes them stick together when contact is made. The newly published work of Li et al. revealed that the combination coagulation of FeCl3 (0.12 mg/L) and polyferric chloride (18 mg/L) can effectively remove ARGs of sulI and sulII, tetO, tetW and tetQ, led to a 0.5–3.1 logs reduction of ARGs in WWTP effluent (Fig. 3) [153].
5.3. Biological approachesBiological treatment was considered a core approach of WWTPs for wastewater treatment, and organic pollutants, such as NH3-N, TP, ARB and ARGs, can be efficiently removed by enormous microorganisms. As reported, the ARGs of tetC and tetA were reduced almost 3 logs after the biological treatment of WWTPs [170]. A similar removal trend of tetO and tetW was also reported by Gao et al. [98]. Many abiotic factors such as temperature, pH, conductivity, chemical oxygen demand (COD), biochemical oxygen demand (BOD), and sludge retention time etc., affected percentage distribution of microbial communities and the performance of the biological treatment unit. Since microorganism has lower bioactivity under anaerobic condition than aerobic treatment and the propagation of resistance genes are inhibited [171, 172], thus anaerobic and anoxic treatment are better choice to remove ARGs. Biotic factors, such as selective or non-selective predation, viral lytic and protozoan interaction, also affect the occurrence of ARB/ARGs in bioreactor [173]. However, high antibiotic degradation and biomass quantity in the biochemical tank of WWTPs accelerated the transmission of ARGs [75, 174], which could be proven by the accumulated tetA (0.4–1.5 logs) and tetB (0.5–1.6 logs) after the activated sludge treatment, compared with the influent [156]. As shown in Fig. 4, bacterial community shift during the biological wastewater treatment promoted the ARGs promotion and the more microbial community, the higher potentially for the horizontal gene transfer of ARGs.

|
Download:
|
| Fig. 4. Removal mechanism of ARGs during the operation of the biological approaches. | |
The removal trend of ARGs during biofilms operation was also investigated. Compared with traditional activated sludge processes, biofilms on the biofilm carrier has less residual microorganisms, implying relatively low abundance of ARGs in the effluent. The ARGs of the tetW and tetO observed within the MBR (membrane bio-reactor) effluent were 1–3 logs less than that of the effluent of the conventional WWTPs [155]. Similarly, Börjesson et al. reported that the tetA and tetB were reduced by 0.6-1.5 logs and 0.6-1.3 logs in the trickling filter [156], and was 0.6-1.2 logs for the ARGs of tetM, tetO, tetQ, tetW, sulI, sulII, and intI1 during the aerated filter operation [157]. In addition, the constructed wetlands (CWs) also exhibited a reasonable performance in ARG-reduction [175], which was evidenced by a higher removal efficiency of 45%–99% for tetracycline resistance genes in swine wastewater during vertical up-flow CW operation [176], in which the biodegradation, substrate adsorption and plant uptake had a predominant role (Fig. 4). Generally, oxidation in surface water plays a vital role during surface flow treatment [175]. Simultaneously substrate adsorption has a prominent effect on ARGs removal in vertical upflow constructed wetlands. Smaller packing particles outperformed bigger one in the elimination of ARGs [176].
To sum it up, biological treatment can remove ARGs in wastewater, but sometimes ARGs were just absorbed onto the sewage sludge or biofilm instead of complete degradation [161]. The potential agricultural use of WWTP sludge may result not only in the transfer of resistant bacteria into the environment, but also in the dissemination of ARGs [23]. Potentially, the rational combination of different biological, chemical, and physical means could be useful in harvesting specific potential, thus providing special ability to improve water quality, simultaneously, reducing serious hazards to health and ecosystems.
6. Summary and future outlookOverall, ARGs in WWTPs are mainly derived from the discharge of livestock breeding, antibiotic manufactures, hospitals and households wastewater, as well as rainwater runoff. WWTPs served as not only reservoirs for the collection of antibiotics from numerous sources, but also the main origination for the emission of ARGs in aquatic environments. The bulk removal efficiency of ARGs in sewage WWTPs was related not only to the applied technical and operational parameters and the concentration/characteristics of the ARGs but also the existence of the pollutants of heavy metals, pesticides, herbicides and etc., within wastewaters. Due to the multi-sources of the ARGs of WWTPs, directly identifying the contamination and implementing targeted mitigation strategies was the main challenge for the control of ARGs. Therefore, we recommend the following research directions:
(1) Pay more attention to industries with substantial quality emissions of ARGs (e.g., breeding, pharmaceutical and medical industry, etc.) and the development of new controlling strategies of ARGs pollution. Economical and effective on-site approaches should be encouraged for the emission reduction of ARGs.
(2) A clarification of the ARGs source-sink relationship would significantly promote the pollution control of ARGs. For WWTPs, more data are still required to determine the predominant sources of ARGs and their corresponding risk level. The priority list of the objective ARGs should be provided.
(3) Biodegradation, especially the bioavailable concentration of antibiotics, is the key to efficiently controlling the spread of ARGs. Thus, the correlations between the distribution of ARGs and the bioavailable concentration of antibiotics should be determined. The effect of QACs, triclosan, pesticides and herbicides on ARGs emission should be analysed.
(4) Effective pre-treatment for the removal of antibiotics, heavy metals, pesticides and herbicides, should be enhanced to alleviate their selective pressure on the proliferation of ARGs in WWTPs.
(5) Traditional biological wastewater treatment processes would cause the transference of ARGs to sewage sludge. Thus, determining the fate and impact of ARGs in WWTPs across their complete life cycle, which includes sludge, should be emphasized.
(6) Optimization of the operational parameters of the biological, physical, and chemical wastewater treatment processes via a comparison of the efficiency of different technologies. Soil infiltration or modified constructed wetlands may be suitable choices.
(7) Note that some research reported ARGs in absolute abundance (gene copies/mL), whereas some research reported ARGs in relative abundance (genes/16S rRNA gene copies). We suggest unification of the measure unit.
Declaration of competing interestWe have no conflicts of interest to declare.
AcknowledgmentsThis work was supported by HIT Environment and Ecology Innovation Special Funds (No. HSCJ201611). The funding from the National Nature Science Foundation of China (No. 51878213), and the funding by State Key Laboratory of Urban Water Resource and Environment (No. 2020TS01).
| [1] |
K.J. Forsberg, A. Reyes, B. Wang, E.M. Selleck, M.O. Sommer, Science 337 (2012) 1107-1111. DOI:10.1126/science.1220761 |
| [2] |
CDC, Antibiotic Resistance Threats in the United States, 2013, U.S. Department of Health and Human Services, CDC, Atlanta, GA, 2013.
|
| [3] |
J.M. Blair, M.A. Webber, A.J. Baylay, D.O. Ogbolu, L.J. Piddock, Nat. Rev. Microbiol. 13 (2015) 42-51. DOI:10.1038/nrmicro3380 |
| [4] |
W. Xue, F. Li, Q. Zhou, Bioresour. Technol. 289 (2019) 121632. DOI:10.1016/j.biortech.2019.121632 |
| [5] |
J. O'Neill, Rev. Antimicrob. Resist. 20 (2014) 1-16. |
| [6] | |
| [7] |
Q. Zhang, G. Tian, R. Jin, Appl. Microbiol. Biot. 102 (2018) 8261-8274. DOI:10.1007/s00253-018-9235-7 |
| [8] |
J.L. Martinez, Environ. Pollut. 157 (2009) 2893-2902. DOI:10.1016/j.envpol.2009.05.051 |
| [9] |
Y. Zhang, A. Li, T. Dai, et al., Environ. Sci. Technol. 52 (2017) 248-257. |
| [10] |
P. Dong, H. Wang, T. Fang, Y. Wang, Q. Ye, Environ. Int. 125 (2019) 90-96. DOI:10.1016/j.envint.2019.01.050 |
| [11] |
T.U. Berendonk, C.M. Manaia, C. Merlin, et al., Nat. Rev. Microbiol. 13 (2015) 310-317. DOI:10.1038/nrmicro3439 |
| [12] |
C. Pal, J. Bengtsson-Palme, E. Kristiansson, D.J. Larsson, BMC Genomics 16 (2015) 964. DOI:10.1186/s12864-015-2153-5 |
| [13] |
N. Devarajan, A. Laffite, N.D. Graham, et al., Environ. Sci. Technol. 49 (2015) 6528-6537. DOI:10.1021/acs.est.5b01031 |
| [14] |
T. Jäger, N. Hembach, C. Elpers, et al., Front. Microbiol. 9 (2018) e120871. |
| [15] |
W.H. Gaze, S.M. Krone, D.G.J. Larsson, et al., Emerg. Infect. Dis. 19 (2013) e120871. |
| [16] |
N.J. Ashbolt, A. Amezquita, T. Backhaus, et al., Environ. Health Perspect. 121 (2013) 993-1001. DOI:10.1289/ehp.1206316 |
| [17] |
C. Rutgersson, J. Fick, N. Marathe, et al., Environ. Sci. Technol. 48 (2014) 7825-7832. DOI:10.1021/es501452a |
| [18] |
X. Guo, Z. Yan, Y. Zhang, et al., Sci. Total Environ. 612 (2018) 119-128. DOI:10.1016/j.scitotenv.2017.08.229 |
| [19] |
Y. Liang, Y. Li, J. Zhao, et al., J. Pain Res. 10 (2017) 951-964. DOI:10.2147/JPR.S132808 |
| [20] |
C. Chen, J. Am. Soc. Inf. Sci. Technol. 57 (2006) 359-377. DOI:10.1002/asi.20317 |
| [21] |
M. Qiao, G. Ying, A.C. Singer, Y. Zhu, Environ. Int. 110 (2018) 160-172. DOI:10.1016/j.envint.2017.10.016 |
| [22] |
L. Wei, K. Qin, N. Zhao, et al., J. Environ. Sci.-China 51 (2017) 173-180. DOI:10.1016/j.jes.2016.08.004 |
| [23] |
F. Zhu, Y. Lv, J. Li, et al., Chemosphere 252 (2020) 577-588. |
| [24] |
B. Chen, L. Hao, X. Guo, N. Wang, B. Ye, Environ. Sci. Pollut. Res. 22 (2015) 13950-13959. DOI:10.1007/s11356-015-4636-y |
| [25] |
W. Ben, J. Wang, X. Pan, Z. Qiang, Chemosphere 167 (2017) 262-268. DOI:10.1016/j.chemosphere.2016.10.013 |
| [26] |
F. Yang, K. Zhang, S. Zhi, et al., Sci. Total Environ. 651 (2019) 2507-2513. DOI:10.1016/j.scitotenv.2018.10.144 |
| [27] |
Q. Sui, J. Zhang, M. Chen, et al., Environ. Pollut. 213 (2016) 751-759. DOI:10.1016/j.envpol.2016.03.038 |
| [28] |
M. Liu, Y. Zhang, M. Yang, et al., Environ. Sci. Technol. 46 (2012) 7551-7557. DOI:10.1021/es301145m |
| [29] |
J.J. Gonzalez-Plaza, K. Blau, M. Milakovic, et al., Environ. Int. 130 (2019) 104735. DOI:10.1016/j.envint.2019.04.007 |
| [30] |
W. Zhai, F. Yang, D. Mao, Y. Luo, Environ. Sci. Pollut. Res. 23 (2016) 12030-12038. DOI:10.1007/s11356-016-6350-9 |
| [31] |
E. Szekeres, A. Baricz, C.M. Chiriac, et al., Environ. Pollut. 225 (2017) 304-315. DOI:10.1016/j.envpol.2017.01.054 |
| [32] |
C. Li, J. Lu, J. Liu, et al., Environ. Sci. Pollut. Res. 23 (2016) 15111-15121. DOI:10.1007/s11356-016-6688-z |
| [33] |
Q. Wang, P. Wang, Q. Yang, Sci. Total Environ. 621 (2018) 990-999. DOI:10.1016/j.scitotenv.2017.10.128 |
| [34] |
J. Li, W. Cheng, L. Xu, P.J. Strong, H. Chen, Environ. Sci. Pollut. Res. 22 (2015) 4587-4596. DOI:10.1007/s11356-014-3665-2 |
| [35] |
R. Aali, M. Nikaeen, H. Khanahmad, et al., Fresen. Environ. Bull. 23 (2014) 2560-2566. |
| [36] |
E. Garner, R. Benitez, E. von Wagoner, et al., Water Res. 123 (2017) 144-152. DOI:10.1016/j.watres.2017.06.046 |
| [37] |
S. Caucci, A. Karkman, D. Cacace, et al., FEMS Microbiol. Ecol. 92 (2016) fiw060. DOI:10.1093/femsec/fiw060 |
| [38] |
W. Sim, J. Lee, E. Lee, et al., Chemosphere 82 (2011) 179-186. DOI:10.1016/j.chemosphere.2010.10.026 |
| [39] |
J.A. Pempek, E. Holder, K.L. Proudfoot, M. Masterson, G. Habing, J. Dairy Sci. 101 (2018) 4473-4478. DOI:10.3168/jds.2017-14055 |
| [40] |
Q. Zhang, G. Ying, C. Pan, Y. Liu, J. Zhao, Environ. Sci. Technol. 49 (2015) 6772-6782. DOI:10.1021/acs.est.5b00729 |
| [41] |
K. Liu, J. Han, S. Li, et al., Ecotox. Environ. Safe. 172 (2019) 451-459. DOI:10.1016/j.ecoenv.2019.01.109 |
| [42] |
L. He, Y. Liu, H. Su, et al., Environ. Sci. Technol. 48 (2014) 13120-13129. DOI:10.1021/es5041267 |
| [43] |
L. Zhou, G. Ying, R. Zhang, et al., Environ. Sci.-Proc. Imp. 15 (2013) 802-813. |
| [44] |
Q.Q. Zhang, G.G. Ying, C.G. Pan, Y.S. Liu, J.L. Zhao, Environ. Sci. Technol. 49 (2015) 6772-6782. DOI:10.1021/acs.est.5b00729 |
| [45] |
P. Gao, D. Mao, Y. Luo, et al., Water Res. 46 (2012) 2355-2364. DOI:10.1016/j.watres.2012.02.004 |
| [46] |
S. Rong, Y. Sun, Z. Zhao, Chin. Chem. Lett. 25 (2014) 187-192. DOI:10.1016/j.cclet.2013.11.003 |
| [47] |
F.A. Khan, B. Soderquist, J. Jass, Front. Microbiol. 10 (2019) 688. DOI:10.3389/fmicb.2019.00688 |
| [48] |
Y. Zhang, C. Zhang, D.B. Parker, et al., Sci. Total Environ. 463 (2013) 631-638. |
| [49] |
J. Liu, Z. Zhao, J.J. Avillan, et al., Environ. Pollut. (2019) 113058. |
| [50] |
J.P. Brooks, A. Adeli, M.R. McLaughlin, Water Res. 57 (2014) 96-103. DOI:10.1016/j.watres.2014.03.017 |
| [51] |
W. Cheng, H. Chen, C. Su, S. Yan, Environ. Int. 61 (2013) 1-7. DOI:10.1016/j.envint.2013.08.023 |
| [52] |
F. Yang, K. Zhang, S. Zhi, et al., Sci. Total Environ. 651 (2019) 2507-2513. DOI:10.1016/j.scitotenv.2018.10.144 |
| [53] |
Y.M. Awad, S. Kim, S.A.A. El-Azeem, et al., Environ. Earth Sci. 71 (2014) 1433-1440. DOI:10.1007/s12665-013-2548-z |
| [54] |
N. Peak, C.W. Knapp, R.K. Yang, et al., Environ. Microbiol. 9 (2007) 143-151. DOI:10.1111/j.1462-2920.2006.01123.x |
| [55] |
Q. Sui, J. Zhang, J. Tong, M. Chen, Y. Wei, Environ. Sci. Pollut. Res. 24 (2017) 9048-9057. DOI:10.1007/s11356-015-5891-7 |
| [56] |
C.A. Biggs, O.I. Olaleye, L.F. Jeanmeure, et al., Environ. Technol. 32 (2011) 133-144. DOI:10.1080/09593330.2010.490852 |
| [57] |
Y.M. Awad, S. Kim, S.A.M. Abd El-Azeem, et al., Environ. Earth Sci. 71 (2014) 1433-1440. DOI:10.1007/s12665-013-2548-z |
| [58] |
W.Y. Xie, Q. Shen, F. Zhao, Eur. J. Soil Sci. 69 (2018) 181-195. DOI:10.1111/ejss.12494 |
| [59] |
S. Shao, Y. Hu, J. Cheng, Y. Chen, Crit. Rev. Biotechnol. 38 (2018) 1195-1208. DOI:10.1080/07388551.2018.1471038 |
| [60] |
E. Kristiansson, J. Fick, A. Janzon, et al., PLoS One 6 (2011) e17038. DOI:10.1371/journal.pone.0017038 |
| [61] |
J. Huang, H. Hu, S. Lu, et al., Environ. Int. 42 (2012) 31-36. DOI:10.1016/j.envint.2011.03.001 |
| [62] |
S. Zheng, C. Cui, Q. Liang, X. Xia, F. Yang, Chemosphere 81 (2010) 1159-1163. DOI:10.1016/j.chemosphere.2010.08.058 |
| [63] |
X. Guo, Z. Yan, Y. Zhang, et al., Sci. Total Environ. 612 (2018) 119-128. DOI:10.1016/j.scitotenv.2017.08.229 |
| [64] |
N. Guo, Y. Wang, T. Tong, S. Wang, Water Res. 133 (2018) 79-86. DOI:10.1016/j.watres.2018.01.020 |
| [65] |
L. Meng, X. Li, X. Wang, et al., J. Environ. Sci.-China 61 (2017) 110-117. DOI:10.1016/j.jes.2017.09.020 |
| [66] |
S. Cheng, Y. Lee, C. Kuo, T. Wu, Int. Biodeter. Biodegr. 102 (2015) 398-401. DOI:10.1016/j.ibiod.2015.04.018 |
| [67] |
B. Wang, G. Li, C. Cai, J. Zhang, H. Liu, Sci. TotalEnviron. 636 (2018) 1463-1469. |
| [68] |
J. Li, W. Cheng, L. Xu, P.J. Strong, H. Chen, Environ. Sci. Pollut. Res. 22 (2015) 4587-4596. DOI:10.1007/s11356-014-3665-2 |
| [69] |
Q. Wang, P. Wang, Q. Yang, Sci. Total Environ. 621 (2018) 990-999. DOI:10.1016/j.scitotenv.2017.10.128 |
| [70] |
J. He, A. Hu, M. Chen, Y. Hu, C. Yu, Microbiology/Weishengwuxue Tongbao 39 (2012) 683-695. |
| [71] |
E. Buelow, J.R. Bayjanov, E. Majoor, et al., FEMS Microbiol. Ecol. 94 (2018) fiy087. |
| [72] |
S. Harris, C. Morris, D. Morris, M. Cormican, E. Cummins, Sci. Total Environ. 468- 469 (2014) 1078-1085. |
| [73] |
T. Chonova, J. Labanowski, A. Bouchez, Contribution of Hospital Effluents to the Load of Micropollutants in WWTP Influents, Springer, Cham, Ferrara, 2017, pp. 135-152.
|
| [74] |
K.P. Scott, C.M. Melville, T.M. Barbosa, H.J. Flint, Antimicrob. Agents Chemother. 44 (2000) 775-777. DOI:10.1128/AAC.44.3.775-777.2000 |
| [75] |
S. Rodriguez-Mozaz, S. Chamorro, E. Marti, et al., Water Res. 69 (2015) 234-242. DOI:10.1016/j.watres.2014.11.021 |
| [76] |
E. Meyer, P. Gastmeier, M. Deja, F. Schwab, Int. J. Med. Microbiol. 303 (2013) 388-395. DOI:10.1016/j.ijmm.2013.04.004 |
| [77] |
J.P. Bound, N. Voulvoulis, Environ. Health Persp. 113 (2005) 1705-1711. DOI:10.1289/ehp.8315 |
| [78] |
R. Wise, J. Antimicrob. Chemoth. 49 (2002) 585-586. DOI:10.1093/jac/49.4.585 |
| [79] |
K. Kümmerer, J. Environ. Manage. 90 (2009) 2354-2366. DOI:10.1016/j.jenvman.2009.01.023 |
| [80] |
A. Karkman, T.T. Do, F. Walsh, M.P. Virta, TrendsMicrobiol. 26 (2018) 220-228. |
| [81] |
O. Alam, T. Deng, Int. J. Sci. Res. Sci. Technol. 1 (2015) 128-139. |
| [82] |
E. Zohdi, M. Abbaspour, Int. J. Environ. Sci. Technol. (Tehran) 16 (2019) 1789-1806. DOI:10.1007/s13762-018-2108-x |
| [83] |
U. Rathnayake, H.M. Azamathulla, Sustain. Water Resour. Manag. 3 (2017) 33-40. DOI:10.1007/s40899-017-0084-9 |
| [84] |
R. Pallares-Vega, H. Blaak, R. van der Plaats, et al., Water Res. 161 (2019) 319-328. DOI:10.1016/j.watres.2019.05.100 |
| [85] |
D. Baral, B.I. Dvorak, D. Admiraal, et al., Environ. Sci. Technol. 52 (2018) 9033-9044. DOI:10.1021/acs.est.8b01219 |
| [86] |
A. DiCesare, E.M. Eckert, M. Rogora, G. Corno, Environ. Pollut. 226 (2017) 473-478. DOI:10.1016/j.envpol.2017.04.036 |
| [87] |
D. Baral, B.I. Dvorak, D. Admiraal, et al., Environ. Sci. Technol. 52 (2018) 9033-9044. DOI:10.1021/acs.est.8b01219 |
| [88] |
S.R. Joy, S.L. Bartelt-Hunt, D.D. Snow, et al., Environ. Sci. Technol. 47 (2013) 12081-12088. DOI:10.1021/es4026358 |
| [89] |
B. Davis, G. Birch, Environ. Pollut. 158 (2010) 2541-2545. DOI:10.1016/j.envpol.2010.05.021 |
| [90] |
E. Garner, R. Benitez, E. von Wagoner, et al., Water Res. 123 (2017) 144-152. DOI:10.1016/j.watres.2017.06.046 |
| [91] |
S. Harris, C. Morris, D. Morris, M. Cormican, E. Cummins, Sci. Total Environ. 468- 469 (2014) 1078-1085. |
| [92] |
L. Sörme, R. Lagerkvist, Sci. Total Environ. 298 (2002) 131-145. DOI:10.1016/S0048-9697(02)00197-3 |
| [93] |
D. Kang, L. Li, X.C. Wang, J. Du, Investigation of Cu, Zn, Mn, Cr, Hg and As migration in domestic wastewater treatment plant, 2010 International Conference on Mechanic Automation & Control Engineering, Wuhan, 2010.
|
| [94] |
L.P. Padhye, C.H. Huang, Proc. Water Environ. Feder. 2012 (2012) 3863-3878. DOI:10.2175/193864712811708365 |
| [95] |
W. Gwenzi, K. Musiyiwa, L. Mangori, J. Environ. Chem. Eng. 8 (2018) 102220. |
| [96] |
J. Zheng, R. Gao, Y. Wei, et al., Environ. Pollut. 230 (2017) 648-654. DOI:10.1016/j.envpol.2017.07.025 |
| [97] |
J. Xu, Y. Xu, H. Wang, et al., Chemosphere 119 (2015) 1379-1385. DOI:10.1016/j.chemosphere.2014.02.040 |
| [98] |
P. Gao, M. Munir, I. Xagoraraki, Sci. Total Environ. 421- 422 (2012) 173-183. |
| [99] |
J. Shentu, K. Zhang, D. Shen, M. Wang, H. Feng, Environ. Sci. Pollut. Res. 22 (2015) 13102-13110. DOI:10.1007/s11356-015-4099-1 |
| [100] |
Y. Luo, D. Mao, M. Rysz, et al., Environ. Sci. Technol. 44 (2010) 7220-7225. DOI:10.1021/es100233w |
| [101] |
D. Mao, S. Yu, M. Rysz, et al., Water Res. 85 (2015) 458-466. DOI:10.1016/j.watres.2015.09.010 |
| [102] |
Y. Xu, J. Xu, D. Mao, Y. Luo, Environ. Pollut. 220 (2017) 900-908. DOI:10.1016/j.envpol.2016.10.074 |
| [103] |
P. Gao, S. He, S. Huang, et al., Appl. Microbiol. Biot. 99 (2015) 3971-3980. DOI:10.1007/s00253-015-6404-9 |
| [104] |
X. Ji, Q. Shen, F. Liu, et al., J. Hazard. Mater. 235- 236 (2012) 178-185. |
| [105] |
C.W. Knapp, S.M. Mccluskey, B.K. Singh, et al., PLoS One 6 (2011) e27300. DOI:10.1371/journal.pone.0027300 |
| [106] |
M.V. Riquelme Breazeal, J.T. Novak, P.J. Vikesland, A. Pruden, Water Res. 47 (2013) 130-140. DOI:10.1016/j.watres.2012.09.044 |
| [107] |
L. Rizzo, C. Manaia, C. Merlin, et al., Sci. Total Environ. 447 (2013) 345-360. DOI:10.1016/j.scitotenv.2013.01.032 |
| [108] |
C. Rutgersson, J. Fick, N. Marathe, et al., Environ. Sci. Technol. 48 (2014) 7825-7832. DOI:10.1021/es501452a |
| [109] |
O. Alam, T. Deng, Int. J. Sci. Res. Sci. Technol. 1 (2015) 128-139. |
| [110] |
A. Pruden, R. Pei, H. Storteboom, K.H. Carlson, Environ. Sci. Technol. 40 (2006) 7445-7450. DOI:10.1021/es060413l |
| [111] |
M.S. Smith, R.K. Yang, C.W. Knapp, et al., Appl. Environ. Microbiol. 70 (2004) 7372-7377. DOI:10.1128/AEM.70.12.7372-7377.2004 |
| [112] |
P. Gao, M. Munir, I. Xagoraraki, Sci. Total Environ. 421 (2012) 173-183. |
| [113] |
Q. Cai, J. Hu, Water Res. 140 (2018) 251-260. DOI:10.1016/j.watres.2018.04.053 |
| [114] |
B. Petrie, R. Barden, B. Kasprzyk-Hordern, Water Res. 72 (2015) 3-27. DOI:10.1016/j.watres.2014.08.053 |
| [115] |
S.E. Beekmann, K.P. Heilmann, S.S. Richter, J. García-de-Lomas, G.V. Doern, Int. J. Antimicrob. Ag. 25 (2005) 148-156. DOI:10.1016/j.ijantimicag.2004.09.016 |
| [116] |
S.B. Levy, The Antibiotic Paradox: How Miracle Drugs Are Destroying the Miracle, Springer, Berlin, 2013.
|
| [117] |
Z. Yu, L. Gunn, P. Wall, S. Fanning, Food Microbiol. 64 (2017) 23-32. DOI:10.1016/j.fm.2016.12.009 |
| [118] |
H. Dang, X. Zhang, L. Song, Y. Chang, G. Yang, Mar. Pollut. Bull. 52 (2006) 1494-1503. DOI:10.1016/j.marpolbul.2006.05.011 |
| [119] |
M.J. Bonten, R. Willems, R.A. Weinstein, Lancet Infect. Dis. 1 (2001) 314-325. DOI:10.1016/S1473-3099(01)00145-1 |
| [120] |
E. Rudolph, M. Zomerdijk, K.C.A. Luyben, L. van der Wielen, Fluid Phase Equilibr. 158 (1999) 903-912. |
| [121] |
K. Kempf, F. Schmitt, U. Bilitewski, R. Schobert, Tetrahedron 71 (2015) 5064-5068. DOI:10.1016/j.tet.2015.05.116 |
| [122] |
B. Halling-Sørensen, G. Sengeløv, F. Ingerslev, L.B. Jensen, Arch. Environ. Con. Tox. 44 (2003) 7-16. DOI:10.1007/s00244-002-1234-z |
| [123] |
K. Li, A. Yediler, M. Yang, S. Schulte-Hostede, M.H. Wong, Chemosphere 72 (2008) 473-478. DOI:10.1016/j.chemosphere.2008.02.008 |
| [124] |
Q. Zhou, M.C. Zhang, C.D. Shuang, Z.Q. Li, A.M. Li, Chin. Chem. Lett. 23 (2012) 745-748. DOI:10.1016/j.cclet.2012.01.039 |
| [125] |
X. Guo, W. Pang, C. Dou, D. Yin, Chemosphere 175 (2017) 21-27. DOI:10.1016/j.chemosphere.2017.01.134 |
| [126] |
D. Livermore, Nat. Rev. Microbiol. 2 (2004) 73-78. DOI:10.1038/nrmicro798 |
| [127] |
Y. Yang, C. Xu, X. Cao, H. Lin, J. Wang, Ecotoxicology 6 (2017) 831-840. |
| [128] |
A. Novo, S. Andre, P. Viana, O.C. Nunes, C.M. Manaia, Water Res. 47 (2013) 1875-1887. DOI:10.1016/j.watres.2013.01.010 |
| [129] |
M.S. Wright, G.L. Peltier, R. Stepanauskas, J.V. McArthur, FEMS Microbiol.Ecol. 58 (2006) 293-302. DOI:10.1111/j.1574-6941.2006.00154.x |
| [130] |
C. Baker-Austin, M.S. Wright, R. Stepanauskas, J.V. McArthur, Trends Microbiol. 14 (2006) 176-182. DOI:10.1016/j.tim.2006.02.006 |
| [131] |
X. Ma, N. Guo, S. Ren, S. Wang, Y. Wang, Environ. Int. 126 (2019) 127-133. DOI:10.1016/j.envint.2019.02.002 |
| [132] |
H. Hasman, F.M. Aarestrup, Antimicrob. Agents Chemother. 46 (2002) 1410-1416. DOI:10.1128/AAC.46.5.1410-1416.2002 |
| [133] |
C. Bednorz, K. Oelgeschläger, B. Kinnemann, et al., Int. J. Med. Microbiol. 303 (2013) 396-403. DOI:10.1016/j.ijmm.2013.06.004 |
| [134] |
S. Börjesson, A. Mattsson, P.E. Lindgren, J. Water Health 8 (2010) 247-256. DOI:10.2166/wh.2009.159 |
| [135] |
M. Guo, Q. Yuan, J. Yang, Chemosphere 93 (2013) 2864-2868. DOI:10.1016/j.chemosphere.2013.08.068 |
| [136] |
C.A. Engemann, L. Adams, C.W. Knapp, D.W. Graham, FEMS Microbiol. Lett. 263 (2006) 176-182. DOI:10.1111/j.1574-6968.2006.00419.x |
| [137] |
Q.B. Yuan, M.T. Guo, J. Yang, PLoS One 10 (2015) e119403. |
| [138] |
Y. Zhang, Y. Zhuang, J. Geng, et al., Sci. Total Environ. 512- 513 (2015) 125-132. |
| [139] |
Y. Zhang, Y. Zhuang, J. Geng, et al., Sci. Total Environ. 550 (2016) 184-191. DOI:10.1016/j.scitotenv.2016.01.078 |
| [140] |
N. Li, G.P. Sheng, Y.Z. Lu, R.J. Zeng, H.Q. Yu, Water Res. 111 (2017) 204-212. DOI:10.1016/j.watres.2017.01.010 |
| [141] |
H. Chen, M. Zhang, Environ. Sci. Technol. 47 (2013) 8157-8163. |
| [142] |
M. Munir, K. Wong, I. Xagoraraki, Water Res. 45 (2011) 681-693. DOI:10.1016/j.watres.2010.08.033 |
| [143] |
S. Börjesson, A. Mattsson, P. Lindgren, J. Water Health 8 (2010) 247-256. DOI:10.2166/wh.2009.159 |
| [144] |
H. Chen, M. Zhang, Environ. Sci. Technol. 47 (2013) 8157-8163. |
| [145] |
R. Stepanauskas, T.C. Glenn, C.H. Jagoe, et al., Environ. Sci. Technol. 39 (2005) 3671-3678. DOI:10.1021/es048468f |
| [146] |
P. Gao, S. He, S. Huang, et al., Appl. Microbiol. Biot. 99 (2015) 3971-3980. DOI:10.1007/s00253-015-6404-9 |
| [147] |
Z. Yu, L. Gunn, P. Wall, S. Fanning, Food Microbiol. 64 (2017) 23-32. DOI:10.1016/j.fm.2016.12.009 |
| [148] |
H. Zhang, Q. Ji, L. Lai, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 1129-1132. DOI:10.1016/j.cclet.2019.01.025 |
| [149] |
R. Chuanchuen, K. Beinlich, T.T. Hoang, et al., Antimicrob. Agents Chemother. 45 (2001) 428. DOI:10.1128/AAC.45.2.428-432.2001 |
| [150] |
M.C. Jennings, K.P. Minbiole, W.M. Wuest, ACS Infect. Dis. 1 (2015) 288-303. DOI:10.1021/acsinfecdis.5b00047 |
| [151] |
M. Tandukar, S. Oh, U. Tezel, K.T. Konstantinidis, S.G. Pavlostathis, Environ. Sci. Technol. 47 (2013) 9730-9738. DOI:10.1021/es401507k |
| [152] |
Y. Negreanu, Z. Pasternak, E. Jurkevitch, E. Cytryn, Environ. Sci. Technol. 46 (2012) 4800-4808. DOI:10.1021/es204665b |
| [153] |
J. Su, B. Wei, W. Ou-Yang, et al., Environ. Sci. Technol. 49 (2015) 7356-7363. DOI:10.1021/acs.est.5b01012 |
| [154] |
B. Kurenbach, D. Marjoshi, C.F. Amábile-Cuevas, et al., MBio 6 (2015) e00009-15. |
| [155] |
F. Baquero, J.L. Martínez, R. Cantón, Curr. Opin. Biotech. 19 (2008) 260-265. DOI:10.1016/j.copbio.2008.05.006 |
| [156] |
X.X. Zhang, T. Zhang, M. Zhang, et al., Appl. Microbiol. Biot. 82 (2009) 1169-1177. DOI:10.1007/s00253-009-1886-y |
| [157] |
Q. Chen, L. Chen, J. Qi, et al., Chin. Chem. Lett. 30 (2019) 1214-1218. DOI:10.1016/j.cclet.2019.03.002 |
| [158] |
J. Bao, X. Wang, J. Gu, et al., Bioresour. Technol. 295 (2020) 121997. DOI:10.1016/j.biortech.2019.121997 |
| [159] |
W. Sun, J. Gu, X. Wang, X. Qian, X. Tuo, Bioresour. Technol. 256 (2018) 342-349. DOI:10.1016/j.biortech.2018.02.052 |
| [160] |
J. Lee, J.H. Jeon, J. Shin, et al., Sci. Total Environ. 605- 606 (2017) 906-914. |
| [161] |
L.L. Wei, X.H. Xia, F.Y. Zhu, et al., Water Res. 181 (2020) 115903. DOI:10.1016/j.watres.2020.115903 |
| [162] |
W.A.M. Hijnen, E.F. Beerendonk, G.J. Medema, Water Res. 40 (2006) 3-22. DOI:10.1016/j.watres.2005.10.030 |
| [163] |
C.W. Mckinney, A. Pruden, Environ. Sci. Technol. 46 (2012) 393-400. |
| [164] |
C. Liu, H. Shen, S. Wang, et al., Chin. Chem. Lett. 29 (2018) 1824-1828. DOI:10.1016/j.cclet.2018.10.025 |
| [165] |
S.M. Shaban, I. Aiad, M.M. El-Sukkary, E. Soliman, M.Y. El-Awady, Chin. Chem. Lett. 28 (2017) 264-273. DOI:10.1016/j.cclet.2016.09.010 |
| [166] |
J. Oh, D.E. Salcedo, C.A. Medriano, S. Kim, J. Environ. Sci.-China 26 (2014) 1238-1242. DOI:10.1016/S1001-0742(13)60594-X |
| [167] |
Y. Zhang, Y. Zhuang, J. Geng, et al., Sci. Total Environ. 512- 513 (2015) 125-132. |
| [168] |
J. Li, Y. Li, Z. Xiong, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 2139-2146. DOI:10.1016/j.cclet.2019.04.057 |
| [169] |
L. Rizzo, D. Sannino, V. Vaiano, et al., Appl.Catal.B:Environ. 144 (2014) 369-378. DOI:10.1016/j.apcatb.2013.07.033 |
| [170] |
T. Zhang, M. Zhang, X. Zhang, H. Fang, Environ.Sci.Technol. 43 (2009) 3455-3460. DOI:10.1021/es803309m |
| [171] |
P.Y. Hong, T.R. Julian, M.R. Jumat, Microbial Safety in Water Resources, Frontiers in microbiology, Lausanne, (2018).
|
| [172] |
L.L. Wei, X.Y. An, S. Wang, et al., Bioresource Technol. 244 (2017) 161-269. |
| [173] |
C.M. Manaia, J. Rocha, N. Scaccia, et al., Environ. Int. 115 (2018) 312-324. DOI:10.1016/j.envint.2018.03.044 |
| [174] |
A. Osińska, E. Korzeniewska, M. Harnisz, S. Niestępski, Appl. Sci. 9 (2019) 387. DOI:10.3390/app9030387 |
| [175] |
L. Liu, Y. Liu, Z. Wang, et al., J. Hazard. Mater. 278 (2014) 304-310. DOI:10.1016/j.jhazmat.2014.06.015 |
| [176] |
X. Huang, C. Liu, K. Li, et al., Water Res. 70 (2015) 109-117. DOI:10.1016/j.watres.2014.11.048 |
 2020, Vol. 31
2020, Vol. 31