b State Key Laboratory of Hydraulics and Mountain River Engineering, College of Architecture and Environment, Sichuan University, Chengdu 610065, China
Heavy metal pollution in water has drawn increasing attentions since heavy metals possess potential threats to human beings by accumulating in the organisms [1, 2]. Large number of heavy metals including Cr, Ni and Cu tend to combine with complexing agents (CAs) such as ethylenediaminetetraacetic acid (EDTA) which are widely applied in diverse industries with the rapid development of industry [3-7]. CA complexed component accounts for a large part of heavy metal speciation in wastewater from these industries [8]. For example, more than 60% of Cr(Ⅲ) in tannery wastewater exists in forms of highly stable and soluble Cr (Ⅲ)-organic complexes [9]. Contrast with free heavy metal ions, heavy metal complexes have characteristics of more stable and intricate structure, because CAs have many functional groups such as carboxy, amino, phenolic hydroxyl. It is reported that the stability constant of Cu-EDTA is five orders of magnitude higher than that of Cu(OH)2 [10]. Heavy metal complexes have high mobility, and are more refractory to be removed from wastewater by conventional water treatment methods [11, 12], which have been a pressing matter from aqueous phase.
Various methods have been developed for the removal of heavy metal complexes from wastewater, including membrane separation [13], adsorption [14] and chemical precipitation [15]. Membrane separation technology utilizes the ultrafiltration membranes with structures containing numerous large pores, which can only trap heavy metal ions combined with watersoluble macromolecular polymers or high molecular weight complexes [16]. The adsorption method mainly uses the porosity of adsorbents which play major roles in directly adsorbing heavy metal complexes in wastewater [17]. The chemical precipitation method generally adds chemicals with a higher stability constant for complexing with metal ions, and forms insoluble precipitates, including a sulfide precipitation method and a complexation precipitation method [18]. However, the use of membrane separation treatment process has restrictions because the membrane is ease to be plugged by impurities causing low efficiency [19]. Adsorption method has some inherent limitations, such as difficulty in the regeneration of adsorbents [20]. The disadvantage of chemical precipitation method is associated with high cost due to addition of a lot of reagents. Therefore, more and more researchers pay attention to emerging technologies for decontamination of heavy metal complexes from wastewater.
In the past decades, advanced oxidation processes (AOPs) constitute a promising technology for the treatment of heavy metal complexes due to highly oxidizing ability, including Fenton oxidation [21], electrochemical oxidation [22], photocatalytic oxidation [23] and ozonation oxidation [24]. The AOPs base on the intermediacy of hydroxyl (·OH) and other radicals to destroy metal-binding complexes and then liberate free metal ions, which can be further eliminated by conventional methods such as chemical precipitation. Meanwhile, CAs can be simultaneously oxidized into non-toxic or low-toxic products like water, carbon dioxide (CO2) and inorganic salts [25-27]. The AOPs can not only destruct heavy metal complexes from wastewater, but also achieve the purpose of recovering metals.
Herein, the goal of this review is to present a precise and comprehensive summary concerning various types of AOPs, based on Fenton (like) oxidation, electrochemical oxidation, photocatalytic oxidation, ozonation and discharge plasma oxidation. Furthermore, the review expounds the reaction mechanisms, advantages and applications to the degradation of heavy metal complexes. The challenges and prospects of AOPs for decomposition of heavy metal complex from wastewater are also introduced.
2. Fenton oxidationFenton oxidation is an attractive and effective technology that can degrade large amounts of harmful organic pollutants. Fenton oxidation technology can be divided into homogeneous and heterogeneous (Fenton-like) reactions [28].
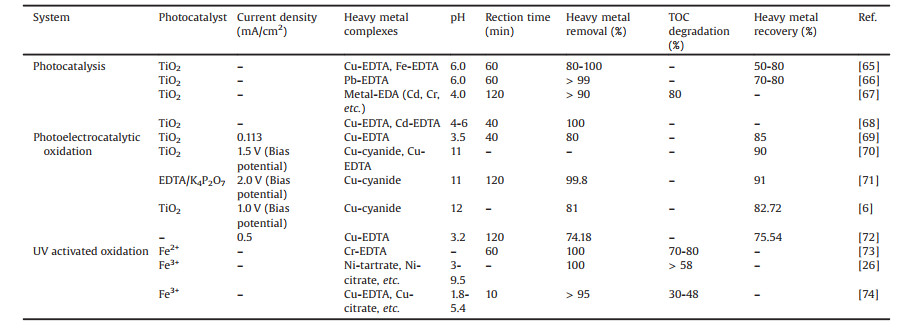
2.1. Homogeneous Fenton oxidationHomogeneous Fenton oxidation employs hydrogen peroxide (H2O2) and ferrous ion (Fe2+) as reactants [29]. As an environmentally friendly oxidant, H2O2 has been extensively utilized as an important reagent in the removal of a huge amount of refractory organics [30]. Typically, H2O2 can generate the production of highly reactive and nonselective ·OH by reacting with Fe2+ at pH near to 3 (Eq. 1). ·OH is a strong oxidant, whose oxidation potential is as high as 2.8 V [31].
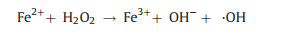
|
(1) |
The mechanism of Fenton oxidation for elimination of heavy metal complexes is to use ·OH to destroy the connection of organic ligands with metal ions and the released organic matters can be subsequently degraded, which is shown in Fig. 1. The released heavy metal ions are usually removed by hydroxide precipitation.
|
Download:
|
| Fig. 1. Mechanistic diagram of Fenton (like) oxidation for heavy metal complex decomposition. | |
Homogeneous Fenton oxidation is recognized as traditional form of AOPs to destruct metal complexes. Ma et al. [32] used in-situ Fenton reaction to depolymerize Cr-polyphenol complexes and confirmed the reaction mechanism. ·OH generated by Fenton reaction preferentially convert Cr(Ⅲ) to Cr(Ⅵ), and then attack organic ligands for degradation. Fu et al. [33] found that 92.8% of Ni2+ removal was achieved during 60 min treatment under the optimal condition of 1 mmol/L Fe2+, 141 mmol/L H2O2 and initial pH value at 3. Wang et al. [21] also found that 99.8% Ni2+ and 93.4% Ni-EDTA were removed by homogeneous Fenton oxidation and the effluent could meet the standard of industries wastewater in China. Lin et al. [34] developed the strategy of coupling microwaveenhanced homogeneous Fenton oxidation with hydroxide precipitation to remove the metal-EDTA in wastewater successfully. More recently, the interior microelectrolysis (IM) employing iron and carbon as the anodic and the cathodic materials is further implanted in homogeneous Fenton process. A large amount of Fe2+ produced in IM is to be used in homogeneous Fenton process. Lan et al. [35] found that the highest removal efficiency of Cu2+ and chemical oxygen demand (COD) reached up 100% and 87% with the optimal operating parameters in IM-Fenton combined system for Cu-EDTA treatment.
Homogeneous Fenton oxidation has several advantages, such as higher degradation efficiency, simple and flexible operation and no need for energy input, because homogeneous Fenton reaction does not require material transfer in different phases [36]. However, the homogeneous Fentonprocess also has obvious drawbacks, e.g., strict requirements for pH, large dosages of agents of catalyst (Fe2+) and production of a large amount of ferric sludge that needs further disposal [37]. Carboxyl and hydroxyl groups in Fenton oxidation may cause heavy metals to coordinate with by-products, and lead to the formation of larger molecular species [32]. These drawbacks have greatly restricted practical applications and targeted solutions should be proposed to further enhance its applicability.
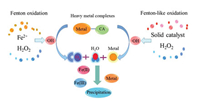
2.2. Fenton-like oxidationFenton-like oxidation, i.e., heterogenous Fenton oxidation develops rapidly nowadays, which employs solid catalysts to enable the lower dissolved metal ions with widened range of pH (Fig. 1) [38]. Avariety of solid catalysts have functions in Fenton-like process for heavy metal complexes destruction, mainly ironbearing materials. Herein pyrite is chosen as an example to illuminate the mechanism of Fenton-like oxidation. Pyrite is oxidized by oxygen or ferric ion (Fe3+) to release numerous ferrous ions and can effectively decompose H2O2, which facilitates the cycle between Fe2+ and Fe3+ [39] (Eqs. 2-4). Meanwhile, ·OH is produced to destruct heavy metal complexes [4]. The released organic ligands are further mineralized into CO2 by ·OH and the free metal ions are eliminated through reduction by aqueous Fe2+ and pyrite (Fig. 1).

|
(2) |
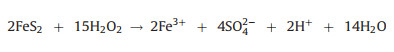
|
(3) |
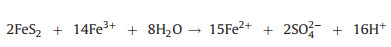
|
(4) |
Fenton-like oxidation weakens pH limit and reduces the sludge production, which is better applicable to water with different acid and alkali conditions. Fu et al. [40] compared Fenton and Fentonlike treatment of Ni-EDTA complexes containing wastewater and found Fenton-like system was more efficient than Fenton one. In addition, with the development of nanomaterials, more and more studies have made use of nanomaterials to the heterogeneous Fenton process [41, 42]. Zero-valent iron was used as catalyst in Fenton-like oxidation to treat Ni-EDTA containing wastewater. The removal efficiency of Ni and COD attained 98.4% and 78.8%, respectively [43]. Liu et al. [44] found that a polymer-supported, nanosized and hydrated Fe(Ⅲ) oxide (HFOD) was effective as a Fenton-like catalyst to remove heavy metal complexes. Cu removal was mainly for the sake of adsorption free Cu2+ and citric acid was degraded to form formic acid, acetic acid and small acid of oxalic acid through decarboxylation process. Liang et al. [45] developed the novel Cuo-CeO2-CoOx composite nanocatalyst to realize the complete decomposition of the Ni(Ⅱ)-citrate complexes in the reaction of 60 min. The spherical Cu2O-Fe3O4@chitosan bifunctional catalyst successfully oxidized Cr(Ⅲ)-organic complexes and effectively inhibited Cr(Ⅵ) accumulation. In the presence of 20 mmol/L H2O2, over 85% of Cr(Ⅲ)-organic complexes were removed in the pH range of 3-6, and the amount of Cr(Ⅵ) remaining in the solution was negligible [46].
Although significant progress has been made for Fenton-like oxidation, it still has the disadvantages in aspects of low water volume treatment capacity and sludge treatment [47]. Catalyst is essentially critical and Fe-bearing catalysts are more promising. Effective strategy to promote the cycling of Fe(Ⅱ) and Fe(Ⅲ) is essential to reduce cost and secondary pollution. Thereby design and exploration of efficient and recyclable catalyst are invariable research hotspots in Fenton-like oxidation field.
3. Electrochemical oxidationFor the purpose of recovery of heavy metal ions from heavy metal complexes, electrochemical oxidation becomes the most popular method (Table 1 [5, 22, 27, 48-53]. Electrochemical processes can be used independently or combined with other AOPs such as Fenton oxidation [54].
|
|
Table 1 Typical studies on heavy metal complex decomposition based on electrochemical oxidation. |
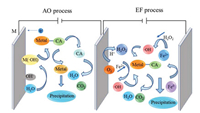
Heavy metal complexes can be destroyed in the anodic area by either anode directly or in situ produced ·OH [55]. The anodic oxidation (AO) process involves following steps: (1) Direct electron transfer to the anode surface M; (2) water or OH‒ in the wastewater is liable to produce powerful physiosorbed ·OH at the anode surface, denoted M(·OH), generated via Eq. 5; (3) strong oxidant ·OH destructs heavy metal complexes (Eq. 6) and free heavy metal ions are deposited on the cathode (Fig. 2).

|
(5) |
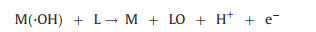
|
(6) |
|
Download:
|
| Fig. 2. Scheme of the electrochemical oxidation used for heavy metal complex degradation. AO: anodic oxidation; EF: Electrochemical Fenton oxidation. | |
The AO has attracted increasing attention for high degradation efficiency, versatility and flexibility [56]. The AO process constitutes a direct and clean way to electrochemically generate ·OH radicals, without adding any chemical reagents or no secondary pollutants. The unique reagent is electrons. Electrode material is vital to AO performance and various combinations of anode and cathode have been tested (Table 1). Guan et al. [57] studied the electrochemical oxidation coupling of electrodeposition processes to treat Ni-ammonia complexes wastewater, which used RuO2/Ti and stainless steel as anode and cathode, respectively. This process removed not only ammonia, but also recovered Ni. Wang et al. [58] developed a novel higee electrochemical reactor with rotating mesh-disc electrodes as cathode to eliminate the electroplating wastewater containing Cu, Ni and Cr complexes. It was an effective method to destroy Cu, Cr and Ni complexes, which reached 99.5%, 97.9% and 98.4% removals within 120 min, respectively.
Nevertheless, oxygen evolution reaction of the anode will compete with the oxidation process of the organic ligand resulting in low treatment efficiency [59]. This inherent drawback restricts actual applications of AO process, whose negative impact can be minimized by novel electrode material development. Applied voltage should also be optimized to reduce operation cost. Furthermore, electrochemical reactors shouldbe better designedfor improving efficiency and saving energy during scaling-up applications.
3.2. Electrochemical Fenton oxidationThe AO process can also be coupled by other AOPs, such as Fenton oxidation, namely electro-Fenton (EF) reaction, which includes electroregeneration of Fe2+ ions in anode (Eq. 7) and the production of H2O2 in cathode. The in-situ generation of H2O2 is derived from cathodic reduction of dissolved oxygen (Eq. 8). Subsequently, Fe2+ reacts with H2O2 to generate strongly oxidative ·OH for decomplexation of heavy metal complexes (Fig. 2).
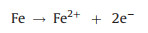
|
(7) |

|
(8) |
EF possesses several major merits compared to classical Fenton reaction. It encompasses: (1) The in situ and continuous production of H2O2 avoiding the hazards during its transport, storage and handling; (2) higher removal efficiency with sustaining production of Fe2+; (3) negligible secondary pollutants and no formation of sludge; (4) effective with high concentration wastewater [60, 61]. The EF process has been recognized as a clean and effective way to decontaminate heavy metal complexes [62]. Zhang et al. [22] demonstrated that synthesized Co-N-doped MoO2 modified carbon felt as cathode resulted in high H2O2 yield by oxygen reduction via two-electron process and 68% of Ni-EDTA was removed within 120 min reaction at pH 3.0 in EF process. Zhao et al. [53] explored the application of electro-Fenton method to simultaneously destruct the coexistence pollutants of Cu/Ni-EDTA in industry effluents. The results showed that the decomplexation of Cu/Ni-EDTA started with the attack of ·OH and the released Cu2+ and Ni2+ promoted the mutual decomposition of H2O2, which led to significant removal efficiency.
Although EF technique does not require an additional oxidant and reduces operating costs, the deposition of heavy metals on the cathode needs to be considered, as the deposited heavy metals often exist in oxide forms, masking the active sites of the cathodic oxygen reduction materials [63]. The concentration of in situ generated oxidant is relatively lower. Therefore, this technology may have certain limitations in practical applications. Operating factors, such as current density, solution pH and aeration rate should be optimized before its practical application.
4. Photochemical oxidationSolar energy as renewable energy is frequently considered in environmental protection because it is abundant, free and non-polluting. Decomposition of heavy metal complexes has also been performed by applying photochemical method [64]. Photochemical oxidation can be used independently or combined with other AOPs (Table 2 [6, 26, 65-74]), including photocatalytic oxidation, photoelectrocatalytic oxidation and UV activated oxidation.
|
|
Table 2 Typical studies on heavy metal complex decomposition based on photochemical oxidation. |
The rationale of photocatalytic oxidation is to use sufficient energy to illuminate photocatalysts, exciting the formation of photogenerated holes and electrons at the semiconductor surface, which can react with chemical species to achieve degradation of heavy metal complexes (Eq. 9) [75, 76]. Besides light energy, photocatalyst functions essentially, among which titanium dioxide (TiO2) with high stability and nontoxicity has been mostly popular [77]. Holes at the valence band of photocatalyst can migrate to the surface and either directly oxidize adsorbed heavy metal complexes or oxidize hydroxide to produce ·OH which then proceeds to destroy the ligands (Eqs. 10-12). The released metal ions are removed by adsorption onto the photocatalyst (Eq. 13) (Fig. 3a).
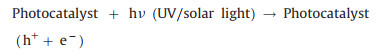
|
(9) |
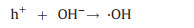
|
(10) |
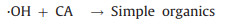
|
(11) |

|
(12) |

|
(13) |

|
Download:
|
| Fig. 3. Scheme of the electrochemical oxidation used for heavy metal complex degradation. AO: anodic oxidation; EF: Electrochemical Fenton oxidation. | |
Benefits of TiO2 photocatalysis for the treatment of metal organic complex include: (1) Heavy metal complex is destructed; (2) the organic ligand is mineralized into CO2, NH3 and other simple organic intermediates; (3) the released metal ions are subsequent removed via adsorption onto the photocatalyst [78, 79]. Photocatalytic oxidation is widely applied in the removal of heavy metal complexes. Rhoads et al. [65] used TiO2 photocatalysis to degrade Cu-EDTA, and Cu-EDTA removal efficiency and Cu recovery efficiency reached 100% and 61% within 60 min operation, respectively. Vohra et al. [66] also developed photocatalytic oxidation with TiO2 photocatalysis 60% of Pb-EDTA was removed in a 60-min duration.
Development of promising photocatalyst is the main effort that researchers focus on. Although TiO2 has advantages, it can only work under UV light condition [80]. The photogenerated electrons and holes are easily recombined. Photocatalyst with visible light response and prevention of recombination of electrons and holes is of great interest. The photocatalyst may flow away with the effluent or deposit with heavy metals. Therefore, reduced loss and inactivation for photocatalyst during applications are essentially important.
4.2. Photoelectrocatalytic oxidationPhotoelectrocatalytic oxidation, birthed from the combination of electrocatalysis and photocatalysis process, has received much attention recently [81]. Photocatalyst is localized on conductive substrate as the anode and an external bias potential is applied to drive the photogenerated electrons to the cathode, thereby delaying the recombination of electron-hole pairs (Fig. 3b). Photoelectrocatalytic oxidation is impressive and has been effectively used to break heavy metal complexes. Similar to the photocatalytic process, ·OH has a great effect on the decomposition of heavy metal complexes, which is generated by reaction of photogenerated holes on the anode with water molecules. In photoelectrocatalytic process, photocatalysis breaks down the connection of heavy metal complexes and releases heavy metals which then deposit on cathode by relying on the strong oxidizing properties of ·OH [82].
There is no need to recycle the photocatalyst which avoids the loss of catalyst because the photocatalytic material is fixed on the conductive substrate in photoelectrocatalytic oxidation. Meanwhile, photoelectrocatalytic process promotes the separation of the photoexcited charge carriers and increases the lifetime of holes under the applied voltage, which greatly improves its performance. It has been regarded one of the most promising techniques for effective destruction of heavy metal complexes. Chaudhary et al. [83] compared different AOPs for Cu-EDTA decomposition. It was found that the electrochemical oxidation alone could efficiently recover Cu, but failed to completely mineralize of EDTA. In contrast, photocatalytic oxidation was capable of achieving degradation of EDTA, but left heavy metals in the bulk solution. The efficiency of Cu recovery and EDTA degradation was 90% and 96% through photoelectrocatalytic oxidation. Zhao et al. [84] also used photoelectrocatalytic process to remove Ni-EDTA in comparison with individual photocatalytic and electrooxidation process, which included that photoelectrocatalytic method was the most effective, resulting in 45% of Ni and nearly 90% of total Ni-EDTA being removed within 180 min. Furthermore, oxidants can also be added to photoelectrocatalytic process to further improve the efficiency [85, 86]. Zeng et al. [87] explored the improvement of Cu-EDTA decomplexation and simultaneous Cu recovery in photoelectrocatalytic system assisted by H2O2/Cl-. The degradation percentage of Cu-EDTA was 97.63%, and the Cu recovery percentage was 97.66% within 60 min. Similarly, removal efficiency was satisfactory via addition of S2O82- ion into photoelectrocatalytic system. The decomplexation percentage of Cu-EDTA reached 98.4% and the Cu recovery percentage was 98.3% during 60 min [88].
Photocatalyst is also paid special attention to in photoelectrocatalytic oxidation. Besides the properties mentioned in photocatalytic oxidation and architecture of electrochemical system should be considered, the attachment of photocatalyst on anode requires particular concern to prevent abscission and deliver photogenerated electron. The applied external bias potential also needs optimization for reducing energy consumption and photocatalyst damage.
4.3. UV activated oxidationBesides application in photocatalysis process, UV radiation combined with powerful oxidants such as H2O2, persulfate (PS) and chlorine, has been widely employed to remove heavy metal complexes, resulting in various kinds of photochemical AOPs [89, 90]. The elimination of heavy metal complexes is based on free radicals generated during photoexcitation process. The UV/H2O2 process is the most frequently applied, because H2O2 can be photolyzed by UV radiations, leading to the formation of ·OH (Eq. 14) [91]. The UV/PS process has recently attracted significant attention for the destruction of refractory organic pollutants via the generation of sulfate radical (SO4·-) (Eq. 15). SO4·- is a more powerful and selective oxidant, which is more prone to oneelectron oxidation reactions compared with ·OH [92]. The UV/HOCl process is being considered as an alternative technology due to high reduction potential while breaks down into ·OH and chlorine atom [93] (Eq. 16). These reactive radicals play key roles in UV activated oxidation for disposal of heavy metal complexes.
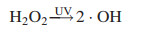
|
(14) |
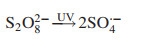
|
(15) |
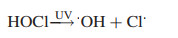
|
(16) |
In the case of UV activated process, the mechanism of heavy metal complex removal is that the radicals generated by photoexcitation attack the heavy metal complex, making heavy metal ions released, and oxidizing the ligands (Fig. 3c). More interestingly, the released heavy metal ions can also catalyze the added oxidants to produce radicals, realizing autocatalytic process [94, 95]. When it is used to treat complexes containing heavy metals with multiple valences, such as Cu, the couple of Cu2+/Cu+ redox cycle can further enhance self-catalytic successive oxidation process [96].
UV activated oxidation process is easy to operate and does not need to deal with secondary pollution caused by TiO2 adsorption, thereby widely accepted for treating wastewater containing heavy metal complexes. Rekab et al. [97] compared the treatment effects of UV/TiO2 and UV/H2O2 technologies on Co-EDTA wastewater, which showed that UV/H2O2 was more effective to the mineralization of EDTA, whereas the UV/TiO2 process was more conducive to the removal of Co2+ by adsorbed on surface of TiO2. Recently, Fe3+ is also considered as a cheap and abundant oxidant in UV activated oxidation, i.e. UV/Fe3+ process, which is efficient to remove heavy metal complexes [10, 98]. Ye et al. [99] successfully reduced the Cr-citrate complex from 10.4 mg Cr/L to 0.36 mg Cr/L along with nearly 60% TOC removal utilizing UV/Fe3+ process.
The combination of oxidants with the UV radiation has resulted into the rapid removal of heavy metal complexes. However, this method still needs in-depth research because the efficient decontamination of heavy metal complexes depend largely on characteristics of metal. For example, UV/H2O2 process causes Pb-EDTA to decompose rapidly in acidic solution with Pb precipitation while the precipitations of Cd-EDTA and Zn-EDTA are ineffective [100]. UV radiation is frequently used for disinfection, which can be combined for decontamination of heavy metal complexes. The installation and placement of UV light source in treatment unit should be also taken into account for efficient illumination.
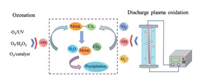
5. OzonationOzone (O3) has a high oxidation potential of 2.07 V and can react directly with organic compounds in the form of molecular O3, or indirectly react with organic compounds through the generation of ·OH during the chain reaction [101]. Based on the strong oxidizing properties of O3, researchers have tried to destroy the heavy metal complexes by ozonation. The principle of the ozonation process is the generation of highly oxidizing radicals [102] (Eqs. 17-19), specially ·OH, capable of oxidizing high recalcitrant contaminants to CO2 and H2O (Fig. 4).
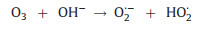
|
(17) |
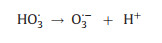
|
(18) |
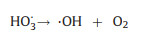
|
(19) |
|
Download:
|
| Fig. 4. Mechanism diagram of ozonation process and discharge plasma oxidation for degradation of heavy metal complexes. | |
Ozonation, one of the commonly used AOPs, is an environmentally friendly technology for wastewater treatment. The ozonation process is emerging as a very promising and alternative wastewater technique which is broadly employed in the field of toxic organics degradation. Ozonation can be carried out at room temperature and pressure without sludge formation, and the residual O3 is also decomposed into water and oxygen. Liu et al. [103] reported that Cu-dimethyl phthalate (DMP) degradation was enhanced by EDTA ozonation, and DMP was completely converted to CO2 and water under the optimal conditions of pH 5.7 and Cu2+/EDTA molar ratio was 3:1. The degradation of Cu/Ni-EDTA in the blended system was a synergistic and continuous decarboxylation process, with Cu2+ and Ni2+ achieving 90% removal even under extremely acidic conditions (pH 3-5) [104]. Huang et al. [105] used ozonation to remove Cu-EDTA, which could completely remove Cu-EDTA within 10 min, and degraded 75%-80% of TOC within 40 min. Moreover, O3 is also coupled with other oxidant, light and catalyst to improve ozonation efficiency, such as O3/H2O2, O3/UV and O3/catalyst processes [106, 107]. Finzgar and Lestan [108] reported that 49.6% and 19.9% of Pb and Zn were actually removed in O3/UV process for Pb-EDTA and Zn-EDTA treatment. When catalyst is supplemented, the behavior of ozonation to degrade heavy metal complex can be further improved [109]. Ozonation can also be integrated with other AOPs. For instance, Zhao et al. [24] found that Ni-EDTA containing wastewater was first treated by Fenton method, and then the effluent was directly used for ozonation treatment, achieving the removal of 99.84% of Ni2+ and 57.13% of TOC.
Ozonation is known for its high efficiency, but still faces some challenges. The selective nature and low solubility of ozone in water and the slow reaction rate lead to low oxidation efficiency [110]. On the other side, the production of ozone consumes energy and increases costs. Ozonation has been widely applied in practical engineering. Catalyst can further improve the performance of ozonation. Effective and immobilized catalyst can broaden the application of ozonation for decomposition of heavy metals complexes. More efforts should be made to improve the efficiencies of O3 utilization and pollutant degradation.
6. Discharge plasma oxidationNon-thermal plasma is an ionized gas with generally equal positive and negative ions produced by means of discharge or ray [111]. It is a new material aggregation state composed of particles such as electrons, ions, ions, molecules and free radicals, and is known as the fourth state substance. The basic principle of heavy metal complex decomplexation is based on various active substances such as ·OH, ·O, H2O2 and O3 [112], when the discharge plasma is triggered (Fig. 4). Discharge plasma oxidation due to its multiple advantages [113]. Non-thermal plasma oxidation has high reactivity and can be operated at normal temperature and pressure without adding additional chemical reagents. Wang et al. [114] used Cu-EDTA as a model pollutant to clarify the removal mechanism by discharge plasma oxidation, and the decomplexation efficiency of Cu complex reached nearly 100%. The findings displayed that 1O2, O2·-, O3 and ·OH produced by discharge plasma were the main contributors for the decomposition of heavy metal complexes, which continuously oxidized to produce low molecular weight products. Cao et al. [115] also obtained similar results and confirmed that O2·-, 1O2, ·OH and O3 caused 87.6%, 80.4%, 74.5% and 4.1% of EDTA-Cu degradation, respectively. Wang et al. [116] pointed out that the Cu removal efficiency reached 80.2% during 60 min after the discharge plasma oxidation treatment for Cu-EDTA solution. A decomposition efficiency of 86.1% was also obtained for Cu-humate through discharge plasma oxidation treatment [117, 118].
Compared with traditional AOPs, discharge plasma oxidation of heavy metal complexes has not been extensively studied. Plasma treatment of heavy metal complexes is still not mature enough, and there are still problems such as high energy consumption and high operating costs. Safety issue during its operation should also be fully considered. These are important aspects that need to be solved urgently.
7. Conclusions and perspectivesHeavy metal complexes have attracted widespread attention in recent years due to high toxicity and difficulty in degradation. In this review, AOPs for decontamination of heavy metal complexes are discussed, including Fenton oxidation, electrochemical oxidation, photocatalytic oxidation, ozonation and discharge plasma oxidation. Discussing the mechanisms, applications, advantages and limitations of each method for removing heavy metal complexes are introduced. These methods can efficiently degrade heavy metal complexes, and some means can even simultaneously recover heavy metals.
Despite the progress that has been made on AOPs for heavy metal complexes decomposition. There are still issues needed to be further studied. Most AOPs are operated under acidic conditions, which requires supplement of large amount of acid resulting in the increase of operation cost. Development of AOPs under near-neutral condition will be more applicable. There are many types of heavy metals and ligands in wastewater, but most existing studies only focus on limited species. The nature of heavy metals and ligands also affects the performance of AOPs, which should be revealed. The realization of resource recovery is a hot topic. Strategies that can recover heavy metals and prevent the inhibition of AOPs performance are very important. Combining different AOPs can achieve unexpected results, which should be concerned. Most AOPs are operated in the laboratory. Scaling up for practical applications should be conducted urgently.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research work was supported by the National Natural Science Foundation of China (NSFC) (No. 41672237) and the Beijing Natural Science Foundation (No. 8192040).
| [1] |
B. Zhang, S. Wang, M. Diao, et al., J. Geophys. Res. Biogeosci. 124 (2019) 601-615. |
| [2] |
J. Shi, B. Zhang, R. Qiu, et al., Environ. Sci. Technol. 53 (2019) 3198-3207. DOI:10.1021/acs.est.8b05053 |
| [3] |
B. Zhang, Z. Wang, J. Shi, H. Dong, Geochim. Cosmochim. Acta 268 (2020) 296-309. DOI:10.1016/j.gca.2019.10.011 |
| [4] |
Y. Ye, C. Shan, X. Zhang, et al., Environ. Sci. Technol. 52 (2018) 10657-10664. DOI:10.1021/acs.est.8b01693 |
| [5] |
L. Li, Z. Huang, X. Fan, et al., Electrochim. Acta 231 (2017) 354-362. DOI:10.1016/j.electacta.2017.02.072 |
| [6] |
S. Tian, C. Dang, R. Mao, X. Zhao, ACS Sustain. Chem. Eng. 6 (2018) 10273-10281. DOI:10.1021/acssuschemeng.8b01634 |
| [7] |
M. Palamarchuk, A. Voit, E. Papynov, et al., J. Hazard. Mater. 363 (2019) 233-241. DOI:10.1016/j.jhazmat.2018.08.080 |
| [8] |
D. Wang, Y. Ye, H. Liu, H. Ma, W. Zhang, Chemosphere 193 (2018) 42-49. DOI:10.1016/j.chemosphere.2017.11.006 |
| [9] |
Y. Tang, J. Zhao, J. Zhou, et al., Water Res. 178 (2020) 115807. DOI:10.1016/j.watres.2020.115807 |
| [10] |
C. Shan, Z. Xu, X. Zhang, et al., Chemosphere 193 (2018) 1235-1242. DOI:10.1016/j.chemosphere.2017.10.119 |
| [11] |
X. Cao, J. Guo, J. Mao, Y. Lan, J. Hazard. Mater. 192 (2011) 1533-1538. DOI:10.1016/j.jhazmat.2011.06.076 |
| [12] |
D. Lestan, C.L. Luo, X.D. Li, Environ Pollut. 153 (2008) 3-13. DOI:10.1016/j.envpol.2007.11.015 |
| [13] |
M.D. Garba, M. Usman, M.A.J. Mazumder, A. Al Ahmed, Inamuddin, Environ. Chem. Lett. 17 (2019) 1195-1208. DOI:10.1007/s10311-019-00861-5 |
| [14] |
L. Wu, H. Wang, H. Lan, H. Liu, J. Qu, Sep. Purif. Technol. 117 (2013) 118-123. DOI:10.1016/j.seppur.2013.06.016 |
| [15] |
X. Xiao, M. Ye, P. Yan, et al., Environ. Sci. Pollut. Res. 23 (2016) 19696-19706. DOI:10.1007/s11356-016-7156-5 |
| [16] |
J. Gao, Y. Qiu, B. Hou, Q. Zhang, X. Zhang, Chem. Eng. J. 334 (2018) 1878-1885. DOI:10.1016/j.cej.2017.11.087 |
| [17] |
X. Li, T. Lu, Y. Wang, Y. Yang, Chin. Chem. Lett. 30 (2019) 2318-2322. DOI:10.1016/j.cclet.2019.05.056 |
| [18] |
C. Peng, L. Chai, C. Tang, et al., J. Environ. Sci. 51 (2017) 222-233. DOI:10.1016/j.jes.2016.06.020 |
| [19] |
S.S. Fiyadh, M.A. AlSaadi, W.Z. Jaafar, et al., J. Clean Prod. 230 (2019) 783-793. DOI:10.1016/j.jclepro.2019.05.154 |
| [20] |
M.J.K. Ahmed, M. Ahmaruzzaman, J. Water Process. Eng. 10 (2016) 39-47. DOI:10.1016/j.jwpe.2016.01.014 |
| [21] |
L. Wang, Z. Luo, J. Wei, et al., Environ. Sci. Pollut. Res. 26 (2019) 29736-29747. DOI:10.1007/s11356-019-05990-6 |
| [22] |
J. Zhang, W. Zhou, L. Yang, Y. Chen, Y. Hu, Environ. Sci. Pollut. Res. 25 (2018) 22754-22765. DOI:10.1007/s11356-018-2373-8 |
| [23] |
L. Zhang, B. Wu, G. Zhang, Y. Gan, S. Zhang, Chem. Eng. J. 358 (2019) 1218-1226. DOI:10.1016/j.cej.2018.10.124 |
| [24] |
Z. Zhao, Z. Liu, H. Wang, W. Dong, W. Wang, Chemosphere 202 (2018) 238-245. DOI:10.1016/j.chemosphere.2018.03.090 |
| [25] |
T. Wang, Q. Wang, H. Soklun, et al., Chem. Eng. J. 370 (2019) 1298-1309. DOI:10.1016/j.cej.2019.04.005 |
| [26] |
B. Zhang, Y. Cheng, J. Shi, et al., Chem. Eng. J. 375 (2019) 121965. DOI:10.1016/j.cej.2019.121965 |
| [27] |
C. Durante, M. Cuscov, A.A. Isse, G. Sandona, A. Gennaro, Water Res. 45 (2011) 2122-2130. DOI:10.1016/j.watres.2010.12.022 |
| [28] |
X. Li, B. Xiao, M. Wu, et al., Chemosphere 245 (2020) 125663. DOI:10.1016/j.chemosphere.2019.125663 |
| [29] |
Y. Fang, A. Deng, Y. Huang, Chin. Chem. Lett. 20 (2009) 1235-1240. DOI:10.1016/j.cclet.2009.05.004 |
| [30] |
B. Shen, C. Dong, J. Ji, M. Xing, J. Zhang, Chin. Chem. Lett. 30 (2019) 2205-2210. DOI:10.1016/j.cclet.2019.09.052 |
| [31] |
A. Babuponnusami, K. Muthukumar, J. Environ. Chem. Eng. 2 (2014) 557-572. DOI:10.1016/j.jece.2013.10.011 |
| [32] |
D. Ma, S. He, C. Shan, et al., Chemosphere 250 (2020) 126214. DOI:10.1016/j.chemosphere.2020.126214 |
| [33] |
F. Fu, B. Tang, Q. Wang, J. Liu, Environ. Chem. Lett. 8 (2009) 317-322. |
| [34] |
Q. Lin, H. Pan, K. Yao, Y. Pan, W. Long, Water Sci. Technol. 72 (2015) 1184-1190. DOI:10.2166/wst.2015.329 |
| [35] |
S. Lan, F. Ju, X. Wu, Sep. Purif. Technol. 89 (2012) 117-124. DOI:10.1016/j.seppur.2012.01.009 |
| [36] |
P. Bautista, A.F. Mohedano, J.A. Casas, J.A. Zazo, J.J. Rodriguez, J. Chem. Technol. Biotechnol. 83 (2008) 1323-1338. DOI:10.1002/jctb.1988 |
| [37] |
L. Wang, Q. Yang, D. Wang, et al., J. Hazard. Mater. 318 (2016) 460-467. DOI:10.1016/j.jhazmat.2016.07.033 |
| [38] |
X. Yang, X. Cheng, A.A. Elzatahry, et al., Chin. Chem. Lett. 30 (2019) 324-330. DOI:10.1016/j.cclet.2018.06.026 |
| [39] |
J. Zhu, H. Xian, X. Lin, et al., Geochim. Cosmochim. Acta 228 (2018) 259-274. DOI:10.1016/j.gca.2018.02.050 |
| [40] |
F. Fu, Q. Wang, B. Tang, Chem. Eng. J. 155 (2009) 769-774. DOI:10.1016/j.cej.2009.09.021 |
| [41] |
J. Qu, T. Che, L. Shi, Q. Lu, S. Qi, Chin. Chem. Lett. 30 (2019) 1198-1203. DOI:10.1016/j.cclet.2019.01.021 |
| [42] |
R. Huang, Z. Fang, X. Yan, W. Cheng, Chem. Eng. J. 197 (2012) 242-249. DOI:10.1016/j.cej.2012.05.035 |
| [43] |
F. Fu, L. Xie, B. Tang, Q. Wang, S. Jiang, Chem. Eng. J. 189- 190 (2012) 283-287. |
| [44] |
B. Liu, S. Pan, Z. Liu, et al., J. Hazard. Mater. 386 (2019) 121969. |
| [45] |
H. Liang, K. Xiao, L. Wei, et al., J. Hazard. Mater. 374 (2019) 167-176. DOI:10.1016/j.jhazmat.2019.04.031 |
| [46] |
C. Shen, H. Li, Y. Wen, et al., Chem. Eng. J. 383 (2020) 1385-8947. |
| [47] |
A. Romeroa, A. Santosa, T. Cordero, et al., Chem. Eng. J. 170 (2011) 36-43. DOI:10.1016/j.cej.2011.03.022 |
| [48] |
X. Lei, L. Li, Y. Chen, Y. Hu, Environ. Sci. Pollut. Res. 25 (2018) 11683-11693. DOI:10.1007/s11356-018-1444-1 |
| [49] |
H. Zhou, J. Luo, Y. Chen, Chemosphere 239 (2020) 124743. DOI:10.1016/j.chemosphere.2019.124743 |
| [50] |
W. Guan, B. Zhang, S. Tian, X. Zhao, Appl. Catal. B:Environ. 227 (2018) 252-257. DOI:10.1016/j.apcatb.2017.12.036 |
| [51] |
D. Zhou, Y. Hu, Q. Guo, et al., Environ. Sci. Pollut. Res. 26 (2019) 1015-1025. DOI:10.1007/s11356-016-8216-6 |
| [52] |
J. Li, J. Bai, K. Huang, et al., Chem. Eng. J. 236 (2014) 59-65. DOI:10.1016/j.cej.2013.09.084 |
| [53] |
Z. Zhao, W. Dong, H. Wang, et al., J. Hazard. Mater. 350 (2018) 128-135. DOI:10.1016/j.jhazmat.2018.02.025 |
| [54] |
C. Wang, Y. Lin, T. Zhou, et al., Chin. Chem. Lett. 30 (2019) 2231-2235. DOI:10.1016/j.cclet.2019.08.055 |
| [55] |
C.A. Martinez-Huitle, S. Ferro, Chem. Soc. Rev. 35 (2006) 1324-1340. DOI:10.1039/B517632H |
| [56] |
M.X. Bi, P. Dian, Y.K. Wang, Z.G. Wang, Z.Y. Wang, Chin. Chem. Lett. 28 (2017) 1159-1162. DOI:10.1016/j.cclet.2017.04.030 |
| [57] |
W. Guan, S. Tian, D. Cao, Y. Chen, X. Zhao, Electrochim. Acta 246 (2017) 1230-1236. DOI:10.1016/j.electacta.2017.06.121 |
| [58] |
J. Wang, X. Chen, J. Yao, G. Huang, Int. J. Environ. Sci. 10 (2015) 5726-5736. |
| [59] |
M. Panizza, G. Cerisola, Chem. Rev. 109 (2009) 6541-6569. DOI:10.1021/cr9001319 |
| [60] |
F.C. Moreira, R.A.R. Boaventura, E. Brillas, V.J.P. Vilar, Appl. Catal. B:Environ. 202 (2017) 217-261. DOI:10.1016/j.apcatb.2016.08.037 |
| [61] |
W. He, Y. Liu, J. Ye, G. Wang, J. Mater. Sci.-Mater. Electron. 29 (2018) 14065-14072. DOI:10.1007/s10854-018-9538-6 |
| [62] |
S. Wen, Z. Niu, Z. Zhang, L. Li, Y. Chen, J. Hazard. Mater. 341 (2018) 128-137. DOI:10.1016/j.jhazmat.2017.07.014 |
| [63] |
Y. Song, T. Sun, L. Cang, S. Wu, D. Zhou, Electrochim. Acta 295 (2019) 605-614. DOI:10.1016/j.electacta.2018.10.162 |
| [64] |
P. Salama, D. Berk, Ind. Eng. Chem. Res. 44 (2005) 7071-7077. DOI:10.1021/ie050100j |
| [65] |
K.R. Rhoads, A.P. Davis, J. Environ. Eng. 130 (2004) 425-431. DOI:10.1061/(ASCE)0733-9372(2004)130:4(425) |
| [66] |
M.S. Vohra, A.P. Davis, Water Res. 34 (2000) 952-964. DOI:10.1016/S0043-1354(99)00223-7 |
| [67] |
I.H. Cho, T.J. Park, H.Y. Kim, K.D. Zoh, H.K. Lee, Water Sci. Technol. 2 (2015) 299-304. |
| [68] |
J.K. Yang, A.P. Davis, Environ. Sci. Technol. 35 (2001) 3566-3570. DOI:10.1021/es010563q |
| [69] |
X. Zhao, L. Guo, J. Qu, Chem. Eng. J. 239 (2014) 53-59. DOI:10.1016/j.cej.2013.10.088 |
| [70] |
X. Zhao, J. Zhang, J. Qu, Electrochim. Acta 180 (2015) 129-137. DOI:10.1016/j.electacta.2015.08.103 |
| [71] |
X. Zhao, J. Zhang, M. Qiao, H. Liu, J. Qu, Environ. Sci. Technol. 49 (2015) 4567-4574. DOI:10.1021/es5062374 |
| [72] |
Y. Chen, X. Zhao, W. Guan, et al., Chem. Eng. J. 324 (2017) 74-82. DOI:10.1016/j.cej.2017.05.031 |
| [73] |
X. Huang, X. Wang, D.-X. Guan, et al., Environ. Sci. Pollut. Res. 26 (2019) 8516-8524. DOI:10.1007/s11356-018-04091-0 |
| [74] |
Z. Xu, G. Gao, B. Pan, W. Zhang, L. Lv, Water Res. 87 (2015) 378-384. |
| [75] |
I.H. Cho, I.S. Shin, J.K. Yang, S.M. Lee, W.T. Shin, J. Environ. Sci. Health. 41 (2006) 1027-1041. DOI:10.1080/10934520600620220 |
| [76] |
I.H. Cho, N.H. Lee, J.K. Yang, S.M. Lee, J. Environ. Sci. Health. 42 (2007) 165-167. |
| [77] |
H.H. Do, D.L.T. Nguyen, X.C. Nguyen, et al., Arab. J. Chem. 13 (2020) 3653-3671. DOI:10.1016/j.arabjc.2019.12.012 |
| [78] |
D. Dimitrakopoulou, I. Rethemiotaki, Z. Frontistis, et al., J. Environ. Manage. 98 (2012) 168-174. DOI:10.1016/j.jenvman.2012.01.010 |
| [79] |
X. Guo, J. Duan, C. Li, Z. Zhang, W. Wang, Colloids Surf. A Physicochem. Eng. Asp. (2020) 124931. |
| [80] |
B. Zhang, S. Zou, R. Cai, M. Li, Z. He, Appl. Catal. B:Environ. 224 (2018) 383-393. DOI:10.1016/j.apcatb.2017.10.065 |
| [81] |
X. Cao, X. Zang, X. Zhou, M. Chen, Y. Ding, Chin. Chem. Lett. 29 (2018) 811-814. DOI:10.1016/j.cclet.2017.12.010 |
| [82] |
X. Zhao, L. Guo, B. Zhang, H. Liu, J. Qu, Environ. Sci. Technol. 47 (2013) 4480-4488. DOI:10.1021/es3046982 |
| [83] |
A.J. Chaudhary, J.D. Donaldson, S.M. Grime, M. Hassan, R.J. Spencer, J. Chem. Technol. Biotechnol. 75 (2000) 353-358. DOI:10.1002/(SICI)1097-4660(200005)75:5<353::AID-JCTB221>3.0.CO;2-Y |
| [84] |
X. Zhao, L. Guo, C. Hu, H. Liu, J. Qu, Appl. Catal. B:Environ. 144 (2014) 478-485. DOI:10.1016/j.apcatb.2013.07.038 |
| [85] |
J. Sun, Y. Guo, Y. Wang, et al., Chem. Eng. J. 332 (2018) 312-320. DOI:10.1016/j.cej.2017.09.041 |
| [86] |
K. Wang, G. Liang, M. Waqas, et al., Sep. Purif. Technol. 236 (2020) 116301. DOI:10.1016/j.seppur.2019.116301 |
| [87] |
H. Zeng, S. Tian, H. Liu, B. Chai, X. Zhao, Chem. Eng. J. 301 (2016) 371-379. DOI:10.1016/j.cej.2016.04.006 |
| [88] |
H. Zeng, S. Liu, B. Chai, et al., Environ. Sci. Technol. 50 (2016) 6459-6466. DOI:10.1021/acs.est.6b00632 |
| [89] |
R. Cai, B. Zhang, J. Shi, M. Li, Z. He, ACS Sustain. Chem. Eng. 5 (2017) 7690-7695. DOI:10.1021/acssuschemeng.7b01137 |
| [90] |
H. Zhang, N. Li, Y. Wang, et al., Chemosphere 184 (2017) 932-938. DOI:10.1016/j.chemosphere.2017.06.064 |
| [91] |
R. Qian, Y. Ma, X. Qi, et al., Chin. Chem. Lett. 16 (2005) 1271-1274. |
| [92] |
F. Ghanbari, M. Moradi, Chem. Eng. J. 310 (2017) 41-62. DOI:10.1016/j.cej.2016.10.064 |
| [93] |
J. Fang, Y. Fu, C. Shang, Environ. Sci. Technol. 48 (2014) 1859-1868. DOI:10.1021/es4036094 |
| [94] |
S. Lan, Y. Xiong, S. Tian, J. Feng, T. Xie, Appl. Catal. B:Environ. 183 (2016) 371-376. DOI:10.1016/j.apcatb.2015.10.030 |
| [95] |
Z. Xu, C. Shan, B. Xie, Y. Liu, B. Pan, Appl. Catal. B:Environ. 200 (2017) 439-447. DOI:10.1016/j.apcatb.2016.07.023 |
| [96] |
X. Huang, Y. Wang, X. Li, et al., Environ. Sci. Technol. 53 (2019) 2036-2044. DOI:10.1021/acs.est.8b05346 |
| [97] |
K. Rekab, C. Lepeytre, F. Goettmann, et al., J. Radioanal. Nucl. Chem. 303 (2014) 131-137. |
| [98] |
L. Zhang, B. Wu, Y. Gan, Z. Chen, S. Zhang, J. Hazard. Mater. 382 (2020) 121107. DOI:10.1016/j.jhazmat.2019.121107 |
| [99] |
Y. Ye, Z. Jiang, Z. Xu, et al., Water Res. 126 (2017) 172-178. DOI:10.1016/j.watres.2017.09.021 |
| [100] |
D. Jiraroj, F. Unob, A. Hagege, Water Res. 40 (2006) 107-112. DOI:10.1016/j.watres.2005.10.041 |
| [101] |
R. Zhang, D. Yuan, B. Liu, Chin. Chem. Lett. 26 (2015) 93-95. DOI:10.1016/j.cclet.2014.10.024 |
| [102] |
S.N. Malik, P.C. Ghosh, A.N. Vaidya, S.N. Mudliar, J. Water Process. Eng. 35 (2020) 101193. DOI:10.1016/j.jwpe.2020.101193 |
| [103] |
Y. Liu, Y. Feng, Y. Zhang, et al., J. Hazard. Mater. 366 (2019) 378-385. DOI:10.1016/j.jhazmat.2018.12.003 |
| [104] |
S. Xu, N. Yan, M. Cui, H. Liu, Environ. Sci. Pollut. Res. 27 (2020) 812-822. DOI:10.1007/s11356-019-06900-6 |
| [105] |
X. Huang, Y. Xu, C. Shan, et al., Chem. Eng. J. 299 (2016) 23-29. DOI:10.1016/j.cej.2016.04.044 |
| [106] |
J. Wang, H. Chen, Sci. Total Environ. 704 (2020) 135249. DOI:10.1016/j.scitotenv.2019.135249 |
| [107] |
N. Wardenier, Z. Liu, A. Nikiforov, S.W.H. Van Hulle, C. Leys, Chemosphere 234 (2019) 715-724. DOI:10.1016/j.chemosphere.2019.06.033 |
| [108] |
N. Finžgar, D. Leštan, Chemosphere 63 (2006) 1736-1743. DOI:10.1016/j.chemosphere.2005.09.015 |
| [109] |
Z. Guan, Y. Guo, S. Li, et al., Sci. Total Environ. 732 (2020) 139223. DOI:10.1016/j.scitotenv.2020.139223 |
| [110] |
T.E. Agustina, H.M. Ang, V.K. Vareek, J. Photochem. Photobiol. C 6 (2005) 264-273. DOI:10.1016/j.jphotochemrev.2005.12.003 |
| [111] |
P. Bruggeman, C. Leys, J. Phys, D: Appl. Phys 42 (2009) 053001. DOI:10.1088/0022-3727/42/5/053001 |
| [112] |
S.B. Gupta, H. Bluhm, Water Sci. Technol. 55 (2007) 7-12. DOI:10.2166/wst.2007.381 |
| [113] |
R. Zhang, D. Yuan, B. Lin, et al., Chin. Chem. Lett. 30 (2019) 826-838. DOI:10.1016/j.cclet.2019.03.051 |
| [114] |
T. Wang, Y. Cao, G. Qu, et al., Environ. Sci. Technol. 52 (2018) 7884-7891. DOI:10.1021/acs.est.8b02039 |
| [115] |
Y. Cao, X. Qian, Y. Zhang, et al., Chem. Eng. J. 362 (2019) 487-496. DOI:10.1016/j.cej.2019.01.061 |
| [116] |
Q. Wang, J. Yu, X. Chen, et al., J. Environ. Manage. 248 (2019) 109237. DOI:10.1016/j.jenvman.2019.07.008 |
| [117] |
T. Wang, L. Zhou, Y. Cao, et al., J. Hazard. Mater. 389 (2020) 121828. DOI:10.1016/j.jhazmat.2019.121828 |
| [118] |
Y. Liu, T. Wang, G. Qu, H. Jia, Sep. Purif. Technol. 248 (2020) 117137. DOI:10.1016/j.seppur.2020.117137 |
 2020, Vol. 31
2020, Vol. 31