The human science and economy have rapid progress to make the global environmental and energy crisis that become an urgent problem. Environmental pollution is a major global issue, which is related to the safety of humans, animals and plants. In recent years, global scientists have paid attention to environmental issues, the concerns allied with harmful and toxic pollutants in water and air bodies. Due to people extracting a lot of resource from nature, manufacturing daily necessities and using medical drug for inflammation, the disposal of their abandoned chemicals has led to contamination in lakes, rivers, oceans and groundwater. Multifarious pollutants are identified in the aquatic environment, such as surfactants, textile dyes, heavy metals, insecticides, and pesticides [1]. Broadly, pollutants can be categorized into inorganic and organic based on the chemical constituents. Inorganic pollutants are typically heavy metals, halides and radioactive wastes etc. And, organic pollutants cover a wide array of pollutants including agrochemicals, pharmaceuticals, dyes, polyaromatic hydrocarbons etc. [2]. Regulators have determined that there is a persistent organic pollutants (POPs) in the organic pollutants that lasts for a long time in the water, and it is easy to accumulate everywhere with the environmental circulation water flow to cause serious pollution. In recent years, there have been many water pollution treatments, such as filtration, reverse osmosis, sedimentation, adsorption, biological and chemical techniques, coagulation, advanced oxidation processes (AOPs) [3-6]. Among these treatment methods, AOPs are potential methods that can transfer harmful organic matter into harmless carbon dioxide (CO2) and water (H2O).
AOPs are powerful methods that were traditionally used for the environmental treatment [7]. Based on their resourcefulness, these methods have received more and more attention around the world and they are becoming more and more versatile. AOPs are a process that based on the in-situ generation of free radicals for oxidizing organic compounds [8]. This majority of available AOPs are based on hydroxyl radicals, which is also a strong oxidant (1.9-2.7 V) but also there are various different AOPs process technologies which have been investigated, such as ozone, UV, electrochemical catalysis and physics based on AOPs [8-13]. Among these AOP methods, the photocatalytic method has attracted the attention to many people due to its advantages of green and environmental protection, and more and more articles have been published in recent years. The photocatalytic activity depends on the light absorption and the photogenerated charge to participate in the chemical reaction. The wider the light absorption range and the prohibition of the recombination of photogenerated electrons and holes, the photocatalytic reaction are facilitated [14-20]. The most researches about photocatalysis are trying to solve the two shortcomings, however, there is only a fewer way to solve these two problems at the same time.
Fortunately, an emerging material has the potential to solve these problems. Carbon quantum dots (CQDs), as a new member in the carbon material family, has been concerned widely due to their excellent optical and chemical properties [21-24]. It inherited the advantages of carbon materials such as non-toxic, harmless, strong electrical conductivity and strong adsorption capacity. In recent studies, it mentioned graphite carbon quantum dots (GQDs) are its cousin, just the graphitization is higher than CQDs. Therefore, GQDs also belong to a kind of highly graphitized CQDs. CQDs have been applied in many application fields: photocatalysis [25, 26], bioimaging [27, 28], biosensing [29, 30], solar cell [31, 32] and drug carriers [33-35]. In these applications, CQDs have successfully demonstrated their excellent light conversion and electron transfer capabilities. This light conversion comes from its unique up-converted photoluminescence (UCPL) effect is due to surface defects, and the electron transfer ability is caused by sp2 hybridization in CQDs [36]. These capabilities can perfectly solve the shortcomings of photocatalysis, thereby improving the photocatalytic activity that can remove organic pollutants in water by CQDs-based photocatalysts.
As shown in the Fig. 1a, there are a lot of reviews on the synthesis, properties, CQDs biological applications and energy conversion, there are few reviews on CQDs and CQDs-based photocatalysts to degrade organic matter in environment. According to the web of science site analysis and search results, there are 28, 675 articles with the keyword is carbon quantum dots, and the number of papers in each year is increasing. It can be seen from Fig. 1b that the research status of CQDs in recent years and how many papers are published in each year, the CQDs researches have increased rapidly every year since 2015. According to a lot of published literatures, the most practical applications have focused on environmental photocatalytic and biological imaging [37]. Therefore, it is necessary to integrate the papers in the environmental purification, conduct a comprehensive analysis of the mechanism, and classify the actual applications. In this paper, we introduce the recent development that ability of CQDs and CQDs-based photocatalysts from the different synthetic routes to remove organic pollutants in water, then review to a mechanism of photodegrading pollutants and discuss the difference between the degradation mechanisms of CQDs and CQDs-based photocatalyst.
|
Download:
|
| Fig. 1. (a) Various photocatalytic applications and synthesis methods of carbon quantum dots. (b) Number of publications per year on carbon quantum dots based photocatalyst from 2014 to 2019 on dated 1 June, 2020. Search engine: web of science, Keyword: Carbon quantum dots. | |
Since Xu et al. accidentally discovered CQDs in 2014, a large number of researchers have turned their attention to CQDs [38]. After people's endeavors in recent years, some preparation methods of CQDs have been invented in order to improve its quantum efficiency and adjust its size. According to precursors and preparation methods, the preparation methods of CQDs can be grouped into two categories: Top-down and bottom-up methods. Here, we will introduce some representative preparation methods for CQDs in detail in Table 1 [39-61].
|
|
Table 1 Comparison of the precursors and synthesis method of a representative CQD preparation. |
Top-down method mainly separates and graphitizes the macromolecules in carbon sources, so that the macromolecules become to small organic substances and then carbonize to form CQDs [39-46]. In Fig. 2a, Das et al. had used different hydrothermal solutions to form GQDs with different heteroatoms (N-GQDs, S-GQDs) [41]. In the Fig. 2b, Saha et al. had stripped out GQDs from GO by a liquid phase method with perchloric acid solution impregnation at 100 ℃ [40]. These two researches' results can be seen that although the precursors are consistent, but different synthesis methods will result in different quantum efficiencies and particle sizes. Top-down method decomposes C-C and C-O in macro carbon materials to form CQDs, so this method can be applied in a wide range. For the preparation of CQDs in waste recycling methods are also increasing [47-49]. Ozin et al. processed black mud and synthesized GQDs in the preparation process. First, the nitric acid (HNO3) and ultrasound is used to decompose large molecules into small molecules, and then small molecules are recarbonized by hydrothermal treatment. The CQDs derived from the black mud have good fluorescence ability, and use its upconversion ability to produce hydrogen with TiO2. Waste recycling CQDs is also a good research by top-down method in the future, which can reduce the cost in the applications.

|
Download:
|
| Fig. 2. (a) Reaction scheme for the synthesis of different types of GQDs with different solvents. (b) Perchloric acid exfoliates GO to form CQDs and fluorescence, TEM data. (c) The possible growth mechanism for CNDs from citric acid and urea under solvothermal condition in DMF. (d) A solvent-engineered MF strategy for high-yield production of multicolor fluorescent CQDs using TNP as active monomers, DMF or EtOH as the single or main solvent, and H2O or CH3COOH as the auxiliary solvent. (e) Characterization of GQDs and of the hybrid GQD_2a by TEM and the size distribution. (f) The different CQDs in PL and UV–vis spectra. (a) Reproduced with permission [41], Copyright 2020, Elsevier. (b) Reproduced with permission [40], Copyright 2017, American Chemical Society. (c) Reproduced with permission [62], Copyright 2016, Wiley-VCH. (d) Reproduced with permission [57], Copyright 2018, Elsevier. (e) Reproduced with permission [22], Copyright 2020, Elsevier. (f) Reproduced with permission [49], Copyright 2019, Wiley VCH. | |
Bottom-up method is the opposite of Top-down method, in which smaller molecules are polymerized to form small molecules, and then carbonized to form CQDs. The advantage of the Bottom-up method is that other substances can be compounded at will and convenient to form new CQDs with specific functions. In this case, the synthesis method of various CQDs is more flexible. Shen et al. [62] citric acid and urea to synthesize surface metal-cation functionalized CNDs with orange emission (580 nm) under solvothermal conditions in N, N-dimethylformamide (DMF) in Fig. 2c, it shows that the first is to polymerize urea and citric acid together through H and C = O, -OH connection, and then added to DMF solution thermal carbonization to form CQDs. From the Fig. 2d, Wu et al. [57] used 1, 3, 6-trinitropyrene (TNP) as active monomers immersed in different solutions and heated at 230 ℃ for 12 h to prepare CQDs. These different solution engineering prepared CQDs which have different excitation band gaps and different molecular structures and will produce light excited by different wavelengths. The above are the two basic preparation methods to make CQDs. Each method has its own advantages and disadvantages. Through this review we can conclude that different preparation methods will synthesize CQDs have different functionalities, we can choose the method according to our applications and conditions. Figs. 2e and f show some methods for identifying CQDs, and only transmission electron microscopy (TEM) and photoluminescence (PL) can directly analyze the existence of CQDs. For X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR), the test results are similar to other carbon materials, it can be seen that a large number of small particles with a size of less than 10 nm by TEM in Fig. 2e. TEM is the most reliable method for the identification of CQDs. PL is also a CQDs authentication method, but it is not an exact method in the Fig. 2f. When the PL of CQDs is excited by incident light, it may then emit light of the different wavelengths. However, the excitation light of different color PL in CQDs obtained by different heteroatom doping and preparation methods, therefore, the some CQDs with low quantum efficiency is no obvious PL effect.
Are different functional CQDs have their ability and role in removing pollutants in water, will it affect the effect of CQDs on photocatalytic degradation in water? For these queries, we will discuss these in the below article.
3. CQDs-based catalysts degrade pollutants in waterIn recent years, photocatalysis has also seen everyone's vision with the continuous development of AOP technology. Moreover, it has the potential for environmental restoration technology, so it has attracted much attention. In the photocatalysis process, ultraviolet light (UV), visible or near infrared light excite photogenerated electrons depending on the band gap [63]. Furthermore, the semiconductor excites electrons (e-) from the valence band (VB) to the conduction band (CB), and generating holes (h+) in the VB and the e- and h- generated to participate the redox reaction in this process [64]. Hence, the recombination ability of h+ and e- effects the photocatalysis ability. It is particularly coincident that CQDs have unique fluorescence and excellent electron transfer capabilities, which can perfectly solve the problems of e- and h+ recombination. Therefore, a large number of CQDs-based photocatalysts have emerged in energy conversion and environmental remediation applications in recent years.
3.1. CQDs as potential photocatalytic materialA large number of studies have proved that CQDs can independently degrade organic pollutants and sterilize water. Due to the particle size of CQDs less than 10 nm has quantum size effect, it will produce lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) similar to VB and CB of photocatalyst, and the size of the band gap will also change with the CQDs particle size, so CQDs will have a redox capacity similar to photocatalysis [65, 66]. In the Table 2 [65, 67-73], it counted the treatment of environmental pollution by CQDs in previous studies, and analyzed the preparation process of CQDs, the effect of removing pollutants and its role in the removing pollutants process. A lot of researches have been conducted on CQDs and heteroatoms CQDs.
|
|
Table 2 Previous researches in the application of CQDs for exclusion of impurities present in environment. |
First, Chen et al. [70] produced the GQDs are stripped from GO and carbon nanotube (CNT). During the process, photogenerated electrons and holes would transfer to the surface of CDs, which act as a redox site and react with the adsorbed reactants. These studies show that CQDs have the redox ability for reducing CO2, and the removal rate reaches 74.8% within 5 h. It proves that CQDs have the potential to be applied in environmental purification. CQDs doped with different heteroatoms will cause their particle size and the width of CQDs bandgap to change [41, 66]. Therefore, CQDs doped with heteroatoms also lead to a larger light absorption range, it will further enhance the redox capacity of CQDs. And heteroatom doped CQDs will produce a defect, to capture electrons or trap pollutants by electrostatic adsorption. Dong et al. [65] used sulfurdoped CQDs to remove Basic fuchsin in water under visible light, and the removal rate reached 81% in 2 h. Esmaiel et al. [71] used Ndoped CQDs to increase the range of the photocatalytic absorption spectrum so that its photogenerated electrons can be excited in UV, visible light and near infrared light (NIR) to remove methylene blue reached 99% within 150 min. By comparing the effect of pure CQDs and heteroatom-doped CQDs in removing pollutants, the addition of heteroatoms significantly improves removing pollutants. The above studies doped non-metal atoms with CQDs, however, it doping with metal ions will also improve their redox ability. Jiang et al. [69] prepared a kind of double heteroatom doped Cu, N-CQDs, the Cu existence will produce a photothermal effect in this system. In photooxidative degradation, CQDs have the ability of photothermal degradation, and the ability to kill cells reaches 80% within 6 h under NIR. Wu et al. double-doped CQDs with metal ions (FeCuCDs), especially for their Fe and Cu dopants chelated with the graphene structure, play as electron acceptor and electron donor in the photocatalysis. The removal effect of diatomic doping CQDs for 1, 4-DHP has been greatly improved that reaches 94% within 100 min under visible light. The above researches show that CQDs can be used as a photocatalyst to remove environmental pollution, and the removal effects of some heteroatom-doped CQDs are closer than these common photocatalysts. It shows that CQDs will become a photocatalyst with potential and development value.
3.2. CQDs based photocatalytic materialCQDs can not only remove contaminants by themselves, but also it can improve the effect of photocatalyst after being compounded with other photocatalysts. Due to the excellent optical and electrical properties of CQDs, it is a very good auxiliary material for photocatalysis. We integrate a lot of researches for CQDs composite materials in the past few years, which is found in Table 3 [74-97].
|
|
Table 3 Previous researches in the application of CQDs based photocatalysts for exclusion of impurities present in environment. |
The researches of CQDs as a cocatalyst is particularly numerous, and the roles are mainly divided into two aspects: the first is the up-conversion effect, and the second is the electron transport capability or both. The up-conversion effect of CQDs is aimed at the problem that the main catalyst cannot respond under visible and NIR light, up-conversion can convert long-wavelength light to short-wavelength light. Then the emitted light excites the main catalyst to produce a redox effect. Suib et al. [91] prepared CQDs/ TiO2 that this material has the ability to degrade MB under visible light reaches 94.8% within 100 min, it shows that the addition of CQDs plays a decisive role in the degradation of MB by TiO2 under visible light. Li et al. [89] also compounded CQDs with TiO2, it was found that when irradiating CQDs with λ > 500 nm, the emit light with a wavelength λ < 500 nm and the effect of removing MB is 100% within 30 min under visible light. There is another way is saying that CQDs and UV photocatalysts have the ability to degrade under visible light. Huo et al. [84] used CQDs and hallosite nanotube to prepare 3D burger-like CQDs@ZnO@HNT, they believe that the role of CQDs is to reduce the band gap of ZnO, the band gap is adjusted to below 3 eV that makes ZnO excited to produce the ability to degrade tetracycline under visible light, the effect of degrading TC reached 94.28% within 90 min. This creates two different perspectives on the role of CQDs, up-conversion and adjust band gap, we will discuss this issue in detail in Section 4. The second role of CQDs is the electron transport ability, due to the sp2 hybridization in the body makes the electron transport is fast. This ability will inhibit the recombination of electrons and holes, and shorten the path of electrons and contaminants. Zhao et al. [74] prepared CQDs/C3N4 complex material by solution heat method, and the degradation effect of tetracycline reached 82.1% within 80 min, which was 11.4 times the pure C3N4. This fully demonstrates that CQDs have excellent ability to transfer electrons and prevent recombination of electrons and holes. Liu et al. [81] produced N-CQDs/C3N4 to degrade indomethacin in water, this study simultaneously used the two roles of CQDs, and the degradation effect reached 95.1% in 90 min, which was 13.6 times that C3N4. It mentions that C3N4 is excited by light from 420 nm to 550 nm, and then light greater than 550 nm is converted into UV and light less than 550 nm by CQDs. Therefore, the photocatalytic effect of CQDs/C3N4 is stronger than that of pure C3N4. The above researches show that CQDs have a positive effect on the degradation of pollutants by photocatalysts, and they have greatly improved the original photocatalyst. So CQDs is a partner with great potential for photocatalysis.
3.3. CQDs based Z-scheme photocatalytic materialIn recentyears, inorder toimprove thephotocatalytic activityand the ability to separate electrons and holes, researchers have vigorously pursued the conductivity of two semiconductor heterojunctions. Particularly, Z-scheme systems as an effective way to improve the photocatalytic degradation activity of one-component catalysts, it has attracted great attention. In Z-scheme systems, e- in the higher CB and h+ in the lower VB of the different semiconductors retain strong reduction abilities and oxidation abilities, respectively. Additionally, the isolated photogenerated e- and h+ can effectively reduce the recombination efficiencyof the charge carriers [92].CQDs as an excellent electronic conductor can reduce the resistance between two semiconductors, it can replace Pt and Ag as a Z-type heterojunction conductor. It canbe seenfrom Table 3 that CQDs have been studied as electronic conductors of Z-type photocatalysts. It is undoubtedly that its photocatalytic ability is improved in the entire system, but how much the degradation ability of pollutants in CQDsfree systems is improved? Lu et al. [92] prepared CQD-SnNb2O6/ BiOCl and SnNb2O6/BiOCl to compare the effects of removing benzocaine under the same degradation conditions, and found that the removal rate of CQD-SnNb2O6/BiOCl was 19% higher than SnNb2O6/BiOCl. Moreover, the rate constant of CQD-SnNb2O6/BiOCl degrading benzocaine is twice than the SnNb2O6/BiOCl. Shi et al. [93] synthesized a CdS/CQDs/g-C3N4 composite material and CQDs as up-conversion effect and electron transfer role in this system. This composite material not only improves the photocatalytic degradation of RhB, but also has the effect of degrading RhB under NIR light. Under visible light, CdS/CQDs/g-C3N4 degrades 100% RhB, but the degradation rate of RhB can reach 62% under NIR light. CQDs have both up-conversion and Z-type heterojunction conductor effects. Yuan et al. [96] designed BiVO4/N-CQDs/Cu2O composite for removing tetracycline hydrochloride under visible light in 60 min. Since the CB of BiVO4 is close to the VB of Cu2O, there are CQDs in the middle to help electron transfer. In this way, the composite material will have a higher oxidation potential and reduction potential to make the redox ability stronger. These studies demonstrate the importance of CQDs in the Z-type photocatalytic system, and its excellent electron transfer ability is the key to the Z-type electron transfer path.
The above is the analysis of CQDs combined with other photocatalysts, and their ability to degrade pollutants in three categories. The role and preparation method of CQDs in the photocatalyst system are summarized. Next, the mechanism by which CQDs degrade pollutants will be analyzed, and summarize and classify several mainstream degradation mechanisms in recent years.
4. The mechanism of removing pollutants by CQDs-based photocatalyst 4.1. CQDs degradation mechanismSection 3.1 introduces individual CQDs to degrade pollutants in water, which is very exciting news. In addition to the celebration, we need to further study the mechanism of CQDs to remove pollutants in water. But there are still some controversies about the mechanism of CQDs degradation of pollutants. The researches about the mechanism are roughly divided into two categories: photocatalysis, and photooxidation. Photocatalysis means that CQDs can become an independent photocatalyst, which has all the properties of photocatalysts. It can be seen from Figs. 3a and b that CQDs act as a photocatalyst, which generates photo-generated electrons through photoexcitation, and the photo-generated electrons move from VB to CB. The holes can directly oxidize pollutants, and electrons react to form superoxide radicals with water [65, 72]. And, it was proved that h+ and superoxide radicals are the main oxidative active species through free radical quenching experiments [65]. Moreover, the required band gap width can be adjusted according to the size of the CQDs particle. Fig. 3c shows that CQDs and MB simultaneously act as photocatalysts, forming a type II-specific heterojunction structure. By the light irradiation, the photo-excited electrons move from LUMO band of MB towards the LUMO band of NGQDs and the holes move in opposite direction, this process will degrade MB by trapping electrons from its structure. Therefore, the superoxide radical and hydroxyl radical generated by the reaction oxidatively degrade the pollutants [71]. Photooxidation means that the role played by CQDs is not strictly a photocatalysis, CQDs are only excited by light to form excited states. As can be seen from Fig. 3d, CQDs are excited by NIR from S0 to S1 excited singlet state. CQDs can then sensitize 3O2 to singlet oxygen when passing through the excited triplet on the way back [69]. And through ESR data, it was found that only singlet oxygen appeared in this mechanism, and superoxide radicals and hydroxyl radicals were not found. Singlet oxygen became the main active species. The photooxidation exists the second mechanism is shown in Fig. 3e. First, CQDs are excited by light to form an excited state, and then CQDs radicals are formed. During the process of CQDs change to the excited states, electrons are transferred to the 1, 4-dihydro-2, 6-dimethylpyridine-3, 5-dicarboxylate (1, 4-DHP). The electrons are transferred through the doped Fe and Cu to fully react with the pollutant to generate 1, 4- DHP radical cations. When 1, 4-DHP was gradually consumed after 30 min, electron transfer from CQDs to O2 came to the major role. Then 1, 4-DHP+· can release H+ and its electron transfers to molecular oxygen under air atmosphere, generating an aromatization product of 1, 4-DHP. Subsequently, reactive oxygen radical (O2·) competes with the photosensitizer CQDs-· to form superoxide anion radical (·O2-) and FeCu-CDs anion restore the ground state. Finally, ·O2- reacts with H+ to produce H2O2 [68].
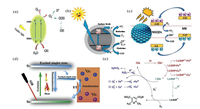
|
Download:
|
| Fig. 3. (a) The possible mechanism of photocatalytic removing BF by the S-GQDs. (b) Mechanism of the electron transfer due to solar energy and decoloration of MB dye. (c) The photocatalytic mechanism of NGQDs. (d) Schematic illustration of photoinduced 1O2 generation by Cu, N-CDs. (e) Mechanism for the photooxidation of 1, 4-DHP with superoxide radical anion. (a) Reproduced with permission [65], Copyright 2018, Elsevier. (b) Reproduced with permission [72], Copyright 2018, Elsevier. (c) Reproduced with permission [71], Copyright 2020, Elsevier. (d) Reproduced with permission [69], Copyright 2018, Elsevier. (e) Reproduced with permission [68] Copyright 2018, Elsevier. | |
These are the two possible mechanisms by which CQDs photodegrade pollutants. For a unified and detailed reaction mechanism, further researches are needed in the future.
4.2. CQDs-based photocatalysts mechanismIn the above discussion, CQDs and photocatalyst composite materials are excellent in degrading pollutants, because CQDs are excellent in electron transfer ability and optical performance. But for the photocatalyst mechanism of CQDs based photocatalyst complex, there are many kinds of statements. Although the reaction mechanism of the complex has been broken with the development of research results, there is no systematic integration. The mechanisms of CQDs based photocatalyst to remove pollutants in water are roughly divided into four types: 1) CQDs as inhibitors to prevent electrons and holes from recombining, 2) CQDs upconversion mechanism, 3) CQDs change the photocatalyst energy band structure and 4) photosensitization mechanism.
First, let's introduce the mechanism of CQDs hindering the recombination of electrons and holes. As can be seen from Fig. 4a, after the photocatalyst is excited by light, electrons and holes will recombine and affect the photocatalytic ability [77, 98]. Fortunately, the addition of CQDs will cause electrons to be transferred to water or react with adsorbed pollutants while being generated. Fortunately, the addition of CQDs will cause electrons to be transferred to water or react with adsorbed pollutants while being generated. The no opportunity for electrons to recombine with holes improves the photocatalytic efficiency, and at the same time improves the effect of holes to oxidize pollutants. In addition to transferring electrons, CQDs can also provide electrons for the photocatalyst, so that the electrons on the conduction band of the photocatalyst and the electrons it provides have photo-reducing ability. In the CQDs/ZnFe2O4 system, the electron transfer way is special direction. CQDs transfer the electrons on ZnFe2O4, and then transfer the electrons excited by CQDs to the conduction band of ZnFe2O4. At the same time, both the electrons on ZnFe2O4 and the electrons excited by CQDs have the ZnFe2O4 reducing ability [80]. The transferred electrons accumulated on the CQDs and were captured by the adsorbed O2 on the ZnFe2O4 surface to form superoxide radicals for removing pollution. These are the mechanisms by which CQDs become electron transferers.
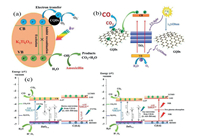
|
Download:
|
| Fig. 4. (a) Schematic illustration of the photocatalytic mechanism for CQDs/K2Ti6O13. (b) Schematic of the photocatalytic CO2-H2O reduction pathways to CO over GQDs/TiO2 under UV/vis light irradiation. (c) Proposed mechanism for CO2 photoreduction on ZnO1-x/C (0.6) under UV/vis light and NIR only irradiation. (a) Reproduced with permission [77], Copyright 2020, Elsevier. (b) Reproduced with permission [49], Copyright 2019, Wiley-VCH. (c) Reproduced with permission [99] Copyright 2018, Elsevier. | |
Second, the mechanism of degradation pollutants in the CQDs based photocatalyst is the up-conversion effect. Since the photocatalyst has the limitation of the absorbed light wavelength, it is necessary to increase the light absorption range. Figs. 4b and c show the mechanism of CQDs photocatalysts with different excitation wavelengths to degrade pollutants. The visible wavelength light is irradiated on the CQDs and the CQDs produce a light with a wavelength smaller than the incident light. This light can excite the photocatalyst that the incident light wavelength cannot excite, and finally achieve the effect of degrading pollutants. There is no definite mechanism for how light conversion is generated by up-conversion. In Fig. 4c, Biswas et al. [99] proposed a possible mechanism for the up-conversion of CQDs. It indicates that the up conversion of CQDs may be caused by two-photons. CQDs first absorb two photons in light with long wavelength and low energy, then emit a photon with high energy and short wavelength. CQDs act as a photoconversion and electron transfer function for photocatalytic degradation. Although there are many studies that prove that CQDs composite photocatalysts degrade pollutants under long-wavelength light is the effect of CQDs up-conversion, but some studies do not think so. Many previous studies have shown that Ti—O—C in TiO2/C composites can shorten the original band gap of TiO2. Therefore, the photocatalysts remove pollutants under long-wavelength light due to the CQDs may be a change the band gap of photocatalysts.
Third, CQDs may change the band gap of the original photocatalyst for removing organic pollutants. As can be seen from Figs. 5a and b, the CQDs loaded onto BiOCl adjust the band gap of the original BiOCl [25, 100]. From the ESR data in the Fig. 5a, it was found that superoxide radicals suddenly appeared after the CQDs were loaded. Therefore, the position of the CB has changed so that the reduction potential is sufficient to generate superoxide radicals, and the VB has also changed to produce a smaller band gap. It can be seen from Fig. 5b that the VB and CB of BiOCl change after CQDs are loaded with BiOCl, resulting in a compound that can degrade CIP under visible light. The superoxide radicals and holes are used as the main active species to degrade pollutants. It can be seen from Fig. 5c that the CQDs loaded TiO2 found that the band gap changes due to the presence of Ti—O—C bonds, and as the particle size of CQDs changes, the band gap of TiO2 decreases more obviously [101]. This mechanism illustrates that CQDs may change the band gap of the photocatalyst.

|
Download:
|
| Fig. 5. (a) Energy band structure of CQDs/BiOCl. (b) Schematic model for the charge separation and CIP photodegradation process over BiOCl/NGQDs photocatalyst under visible-light illumination. (c) UV/vis and energy band structure spectra of GQD–TiO2 composite films. (d) Schematic illustration of the proposed reaction mechanism for photocatalytic reduction of Cr (VI) over 3D CQDs/GA photocatalysts. (e) Schematic illustration of photosensitation-like degradation of organic compounds or DCF. (a) Reproduced with permission [25], Copyright 2020, American Chemical Society. (b) Reproduced with permission [100], Copyright 2019, Elsevier. (c) Reproduced with permission [101], Copyright 2016, The Royal Society of Chemistry. (d) Reproduced with permission [102], Copyright 2018, Elsevier. (e) Reproduced with permission [79], Copyright 2019, Elsevier. | |
Forth, due to CQDs have a photosensitizing effect, there are many studies that CQDs and photocatalyst composites degrade organic pollutants due to the photosensitizing effect of CQDs. As shown in Fig. 5d, the light irradiated to CQDs produces a photosensitizing effect, resulting in the generation of sensitized electrons [102]. Because the CQDs and the photocatalyst have a chemical bond to recombine together, it will cause the electrons to conduct back to the CB of the photocatalyst or reflect the center, which produces the ability to generate superoxide radicals and degrade pollutants. In the Fig. 5e, Liu et al. show a photosensitation-like electronic pathway is different from Fig. 5d without CQDs are activated by light [79]. The DCF is decomposed by the electron from the pollutant itself which is transferred to the CB of C3N4 through CQDs. Then, the activated electrons undergo a reaction pathway similar with the photosensitation process to generate ·O2- and a little of ·OH. This mechanism is different from the traditional photosensitivity is a new sight. These are the four possible mechanisms by which CQDs-based photocatalysts degrade pollutants, and the specific mechanism requires further efforts by researchers in future.
4.3. Mechanism of Z-type electron transfer path through CQDsIn Section 3.3 we introduced that CQDs can be used as conductors in the Z-electron transfer system with two photocatalysts, and the role and mechanism of CQDs as conductors in this system are not described in detail. And why the photocatalytic activity of the Z-type photocatalyst formed by CQDs is better than the several other heterojunction type photocatalysts, we will focus on these potential mechanisms. It is well known that the higher the redox potential, the stronger the redox ability, and the redox potentials of the type I and type II heterojunctions in the photocatalyst are low, although the electron and hole recombination ability are alleviated.
Lu et al. [92] designed CQD-SnNb2O6/BiOCl (CQD-SNO/BOC) Zscheme system, the research introduces that the two catalysts are combined together, which is similar to the original type II heterojunction according to the energy band position in the Fig. 6a. However, through ESR and free radical capture experiments, it was found that SnNb2O6 cannot generate ·OH, as its VB (1.52 eV) is lower than the redox potential of H2O/·OH (2.3 eV vs. NHE), which is contrary to the results of Mott-Schottky spectra. Therefore, a photocatalytic reaction mechanism based on a Zscheme system for the SNO/BOC and CQD-SNO/BOC composites is proposed in Fig. 6a. As expected, the ·O2- produced by the photogenerated electrons in the VB of BiOCl and the ·OH produced by the photogenerated h+ in the CB of SnNb2O6. In order to increase the recombination ability of the electrons on the CB in BiOCl and the holes on the VB in SnNb2O6, CQDs were used as a bridge to transfer the electrons of BiOCl into the holes in SnNb2O6, this will increase the efficiency of photocatalysis compared to Z-type photocatalysts without CQDs. CQDs can not only serve as a conductor, they can also become a true photocatalyst.
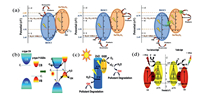
|
Download:
|
| Fig. 6. (a) Possible photocatalytic mechanisms for a conventional SNO/BOC heterojunction, Z-scheme SNO/BOC system and Z-scheme CQD-SNO/BOC system. (b) The possible photocatalytic mechanism of P-GQDs/CN. (c) Schematic illustration of charge transfer process for the GQDs/ZnO NWs under solar light irradiation. (d) Energy band diagram of the Z-scheme electron transfer (CdS/CQDs/g-C3N4) mechanism. (a) Reproduced with permission [92], Copyright 2019, Elsevier. (b) Reproduced with permission [103], Copyright 2018, American Chemical Society. (c) Reproduced with permission [104], Copyright 2016, American Chemical Society. (d) Reproduced with permission [97], Copyright 2020, Elsevier. | |
In Fig. 6b, CQDs as a photocatalyst which are combined with other semiconductors to form p-n heterojunction composites. Dong et al. doped CQDs with P element from n-type semiconductor to p-type semiconductor. The Fermi level of n-type semiconductor or p-type semiconductor is near CB or VB. If two semiconductors are combined, as a result, the bands of the p-type semiconductor would be raised, and bands of the n-type semiconductor would be lowered until their Fermi levels were equal [103]. An energy band structure similar to a Z-type semiconductor will be formed. For example, Moshfegh et al. [104] compounded CQDs with ZnO to form a Z-type heterojunction complex. It can be seen from Fig. 6c that the VB of GQDs is very close to the CB of ZnO, and it is proved to be a Z-type electron transfer path. Yue et al. [105] used the ESR and UV-vis diagrams prove that NCQDs are indirect photocatalysts, and form a Z-type heterojunction with m-Bi2O4. The holes of m- Bi2O4 directly oxidize pollutants, and the electrons of CB on NCQDs generate ·O2-. If the NCQDs are direct transfer semiconductors, the type I heterojunction will form m-Bi2O4 with lower degradation efficiency than Z-type heterojunction. CQDs can not only form a pn heterojunction with another catalyst, but also become a conductor of p-n heterojunction. A p-n heterojunction can be formed between Cu2O and BiVO4, and CQDs act as conductors that promote the p-n type heterojunction change to a Z-type heterojunction [95]. As mentioned earlier, CQDs can convert the wavelength of light through fluorescence. From the Fig. 6d, it is concluded that CQDs first convert light and excite the surrounding photocatalyst, and then form a Z-type heterojunction as an electronic conductor to promote the degradation of pollutants [93, 97]. Since CQDs are the conductors of the Z-type electron transfer path, they are also the way for electrons to contact pollutants [94, 96]. The above conclusion is a discussion on how CQDs become a Z-type heterojunction promoter to promote photocatalytic degradation of pollutants.
5. Conclusion and outlookIn this review, we mainly focus on the preparation of CQDs, CQDs-based photocatalysts, their applications and mechanisms in the environment. CQDs is a potential carbon material, and its electrical conductivity and biocompatibility have been greatly developed in various applications. Due to its superior optical performance and electrical performance, it greatly promotes the ability in the photocatalytic process, and solves the limitations of the photocatalyst under individual conditions. In recent years, there have been more and more studies on CQDs-based photocatalysts to solve the water pollution. This article unifies and discusses its photocatalytic effects and mechanisms. In the purifying environment process by CQDs-based photocatalysts, many CQDs capabilities are utilized, such as tunable PL, unique UCPL and unique photoinduced electron transfer properties, which have a positive effect on photocatalytic applications. In particular, CQDs are able to play multifaceted roles in photocatalysis, such as electron mediators, photosensitizers, spectral converter and sole photocatalyst.
Although significant advancement has been made in this area over the past decade, there are still many issues worthy of further investigation as follows:
(1) The photocatalytic mechanism of CQDs is not yet clear, and there is no definite evidence to prove that CQDs are photocatalysts. The mechanism of CQDs degradation of pollutants in water should be further studied.
(2) CQDs are soluble organic matter, which may cause pollution and increase TOC in the environment. When it is used alone or in combination with a photocatalyst for environmental purification applications, how many CQDs will enter the environment and impact the health of environment.
(3) CQDs are also a kind of soluble organic matter, however, the superoxide free radicals produced by themselves with the excitation of light that will have any effect on their structure and dosage. Therefore, the changes and decomposition of CQDs in the advanced oxidation process are analyzed.
(4) The mechanism of CQDs UCPL is not yet clear. There are many CQDs combined with photocatalysts to degrade pollutants in visible light or NIR light. Most photocatalysts use the upconversion effect of CQDs, but the clear mechanism of UCPL was not proved. So, it is necessary to clear the UCPL mechanism.
(5) CQDs doped with different heteroatoms to have different fluorescence effects, and whether it has an effect on the removal effects and mechanisms of pollutants need to be studied.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 51978201) and the State Key Laboratory of Urban Water Resource and Environment (No. 2020DX08).
| [1] |
S. Sharma, V. Dutta, P. Singh, et al., J. Cleaner Prod. 228 (2019) 755-769. DOI:10.1016/j.jclepro.2019.04.292 |
| [2] |
P. Samanta, A.V. Desai, S. Let, S.K. Ghosh, ACS Sustain. Chem. Eng. 7 (2019) 7456-7478. DOI:10.1021/acssuschemeng.9b00155 |
| [3] |
G. Sharma, A. Kumar, M. Naushad, et al., J. Cleaner Prod. 172 (2018) 2919-2930. DOI:10.1016/j.jclepro.2017.11.122 |
| [4] |
A. Aleboyeh, M.B. Kasiri, M.E. Olya, H. Aleboyeh, Dyes Pigm. 77 (2008) 288-294. DOI:10.1016/j.dyepig.2007.05.014 |
| [5] |
N. Daneshvar, A.R. Khataee, M.H. Rasoulifard, M. Pourhassan, J. Hazard. Mater. 143 (2007) 214-219. DOI:10.1016/j.jhazmat.2006.09.016 |
| [6] |
M.B. Kasiri, H. Aleboyeh, A. Aleboyeh, Appl. Catal. B: Environ. 84 (2008) 9-15. DOI:10.1016/j.apcatb.2008.02.024 |
| [7] |
M.M. M'Arimi, C.A. Mecha, A.K. Kiprop, R. Ramkat, Renew. Sustain. Energy Rev. 121 (2020) 109669. DOI:10.1016/j.rser.2019.109669 |
| [8] |
David B. Miklos, Remy Christian, Jekel Martin, et al., Water Res. 139 (2018) 118-131. DOI:10.1016/j.watres.2018.03.042 |
| [9] |
Y.H. Chuang, A. Szczuka, F. Shabani, et al., Water Res. 152 (2019) 215-225. DOI:10.1016/j.watres.2018.12.062 |
| [10] |
X.Y. Yu, J.L. Sun, G.B. Li, et al., Water Res. 174 (2020) 115622. DOI:10.1016/j.watres.2020.115622 |
| [11] |
J.L. Sun, Y. Chen, Y.Y. Xiang, et al., Chemosphere 228 (2019) 735-743. DOI:10.1016/j.chemosphere.2019.04.168 |
| [12] |
L. Sbardella, I.V. Gala, J. Comas, et al., J. Cleaner Prod. 260 (2020) 121014. DOI:10.1016/j.jclepro.2020.121014 |
| [13] |
N. Wardenier, Z. Liu, A. Nikiforov, S.W.H.V. Hulle, C. Leys, Chemosphere 234 (2019) 715-724. DOI:10.1016/j.chemosphere.2019.06.033 |
| [14] |
Y. Guo, W.X. Shi, Y.F. Zhu, et al., Appl. Catal. B:Environ. 262 (2020) 118262. DOI:10.1016/j.apcatb.2019.118262 |
| [15] |
M.X. Ji, Z.Y. Zhang, J.X. Xia, et al., Chin. Chem. Lett. 29 (2018) 805-810. DOI:10.1016/j.cclet.2018.05.002 |
| [16] |
Y.B. Chen, J.F. Li, P.Y. Liao, et al., Chin. Chem. Lett. 31 (2020) 935-945. |
| [17] |
X.J. Yuan, D. Floresyona, P.H. Aubert, et al., Appl. Catal. B:Environ 242 (2019) 284-292. DOI:10.1016/j.apcatb.2018.10.002 |
| [18] |
R.Q. Zhang, Y.Y. Liu, Z.Y. Wang, et al., Appl. Catal. B:Environ. 254 (2019) 463-470. DOI:10.1016/j.apcatb.2019.05.024 |
| [19] |
M. Humayun, N. Sun, F. Raziq, et al., Appl. Catal. B:Environ. 231 (2018) 23-33. DOI:10.1016/j.apcatb.2018.02.060 |
| [20] |
M.S. Zhu, S. Kim, L. Mao, et al., J. Am. Chem. Soc. 139 (2017) 13234-13242. DOI:10.1021/jacs.7b08416 |
| [21] |
G.J. Huang, Y.Q. Lin, L.X. Zhang, et al., Sci. Rep. 9 (2019) 19651. DOI:10.1038/s41598-019-55996-w |
| [22] |
C.I.M. Santos, L.R. Perez, G. Gonçalves, et al., Carbon 166 (2020) 164-174. DOI:10.1016/j.carbon.2020.04.012 |
| [23] |
H.W. Wang, Y.S. Wang, B. He, et al., ACS Appl. Mater. Interfaces 8 (2016) 18526-18533. DOI:10.1021/acsami.6b03198 |
| [24] |
C.S. Lu, Q.W. Zhu, X.J. Zhang, et al., ACS Sustain. Chem. Eng. 7 (2019) 8542-8553. DOI:10.1021/acssuschemeng.9b00322 |
| [25] |
J. Di, J.X. Xia, M.X. Ji, et al., ACS Appl. Mater. Interfaces 7 (2015) 20111-20123. DOI:10.1021/acsami.5b05268 |
| [26] |
J. Di, J.X. Xia, M.X. Ji, et al., ACS Sustain. Chem. Eng. 4 (2016) 136-146. DOI:10.1021/acssuschemeng.5b00862 |
| [27] |
R. Miao, S.F. Zhang, J.F. Liu, Y. Fang, Chem. Mater. 29 (2017) 5957-5964. DOI:10.1021/acs.chemmater.7b01580 |
| [28] |
D.Y. Yu, L. Wang, H.Y. Zhou, et al., Bioconjugate Chem. 30 (2019) 966-973. DOI:10.1021/acs.bioconjchem.9b00131 |
| [29] |
H.J. Wang, Z.Y. Song, J.J. Gu, et al., ACS Biomater. Sci. Eng. 5 (2019) 4739-4749. DOI:10.1021/acsbiomaterials.9b00583 |
| [30] |
J.Q. Guo, D.F. Liu, I. Filpponen, et al., Biomacromolecules 18 (2017) 2045-2055. DOI:10.1021/acs.biomac.7b00306 |
| [31] |
Y.L. Wang, L.P. Yan, G.Q. Ji, et al., ACS Appl. Mater. Interfaces 11 (2019) 2243-2253. DOI:10.1021/acsami.8b17128 |
| [32] |
M. Ali, R. Riaz, S. Bae, et al., ACS Appl. Mater. Interfaces 12 (2020) 10369-10381. DOI:10.1021/acsami.9b21087 |
| [33] |
Yao Hanchun, Su Li, Zeng Man, et al., Int. J. Nanomed. 11 (2016) 4423-4438. DOI:10.2147/IJN.S108039 |
| [34] |
F.U. Amin, A.K. Hoshiar, T.D. Do, et al., Nanoscale 9 (2017) 10619-10632. DOI:10.1039/C7NR00772H |
| [35] |
N. Mehta Vaibhavkumar, Shiva Shankaran Chettiar, Jigna R. Bhamore, et al., J. Fluoresc. 27 (2016) 1-14. |
| [36] |
S.J. Zhu, Q.N. Meng, L. Wang, et al., Angew. Chem. Int. Ed. 125 (2013) 4045-4049. DOI:10.1002/ange.201300519 |
| [37] |
S. Sharma, V. Dutta, P. Singh, et al., J. Cleaner Prod. 228 (2019) 755-769. DOI:10.1016/j.jclepro.2019.04.292 |
| [38] |
X. Xu, R. Ray, Y. Gu, et al., J. Am. Chem. Soc. 126 (2004) 12736. DOI:10.1021/ja040082h |
| [39] |
Z.A. Qiao, Y.F. Wang, Y. Gao, et al., Chem. Commun. 46 (2010) 8812-8814. DOI:10.1039/c0cc02724c |
| [40] |
S. Maiti, S. Kundu, C.N. Roy, et al., Langmuir 33 (2017) 14634-14642. DOI:10.1021/acs.langmuir.7b02611 |
| [41] |
R. Das, S. Parveen, A. Bora, P.K. Giri, Carbon 160 (2020) 273-286. DOI:10.1016/j.carbon.2020.01.030 |
| [42] |
M.B. Wu, Y. Wang, W.T. Wu, et al., Carbon 78 (2014) 480-489. DOI:10.1016/j.carbon.2014.07.029 |
| [43] |
S.J. Park, J.Y. Park, J.W. Chung, et al., Chem. Eng. J. 383 (2020) 123200. DOI:10.1016/j.cej.2019.123200 |
| [44] |
Z.M. Luo, G.Q. Qi, K.Y. Chen, et al., Adv. Funct. Mater. 26 (2016) 2739. DOI:10.1002/adfm.201505044 |
| [45] |
X. Zhou, Y. Zhang, C. Wang, et al., ACS Nano 6 (2020) 6592. |
| [46] |
Q. Liu, B.D. Guo, Z.Y. Rao, et al., Nano Lett. 13 (2013) 2436. DOI:10.1021/nl400368v |
| [47] |
S. Liu, J.Q. Tian, L. Wang, et al., Adv. Mater. 24 (2012) 2037-2041. DOI:10.1002/adma.201200164 |
| [48] |
S.J. Zhao, M.H. Lan, X.Y. Zhu, et al., ACS Appl. Mater. Interfaces 7 (2015) 17054-17060. DOI:10.1021/acsami.5b03228 |
| [49] |
Z.Y. Jiang, X.H. Zhang, W. Sun, et al., Angew. Chem. Int. Ed. 58 (2019) 14850-14854. DOI:10.1002/anie.201909222 |
| [50] |
L. Wang, S.J. Zhu, H.Y. Wang, et al., ACS Nano 8 (2014) 2541-2547. DOI:10.1021/nn500368m |
| [51] |
Lu Siyu, Sui Laizhi, Liu Junjun, et al., Adv. Mater. 29 (2017) 1603443. DOI:10.1002/adma.201603443 |
| [52] |
J. Zhang, X. Yuan, L. Jiang, et al., J. Colloid Interface Sci. 511 (2018) 296-306. DOI:10.1016/j.jcis.2017.09.083 |
| [53] |
M.K. Barman, B. Jana, S. Bhattacharyya, A. Patra, J. Phys. Chem. C 118 (2014) 20034-20041. DOI:10.1021/jp507080c |
| [54] |
Z. Liang, H.L. Hou, Z. Fang, et al., ACS Appl. Mater. Interfaces 11 (2019) 19167-19175. DOI:10.1021/acsami.9b04059 |
| [55] |
A. Babusenan, B. Pandey, S.C. Roy, J. Bhattacharyya, Carbon 161 (2020) 535-541. DOI:10.1016/j.carbon.2020.01.097 |
| [56] |
Y.X. Zhang, W.D. Zhang, Carbon 145 (2019) 488-500. DOI:10.1016/j.carbon.2019.01.052 |
| [57] |
J. Zhan, B. Geng, K. Wu, et al., Carbon 130 (2018) 153-163. DOI:10.1016/j.carbon.2017.12.075 |
| [58] |
F.F. Zhao, Y.F. Rong, J.M. Wan, et al., Catal. Today 315 (2018) 162-170. DOI:10.1016/j.cattod.2018.02.019 |
| [59] |
X.L. Guo, Z.Y. Ding, S.M. Deng, et al., Carbon 134 (2018) 519-530. DOI:10.1016/j.carbon.2018.04.001 |
| [60] |
H.J. Jian, J. Yu, Y.J. Li, et al., Chem. Eng. J. 386 (2020) 123913. DOI:10.1016/j.cej.2019.123913 |
| [61] |
Y. Ma, A.Y. Chen, Y.Y. Huang, et al., Carbon 162 (2020) 234-244. DOI:10.1016/j.carbon.2020.02.048 |
| [62] |
S.N. Qu, D. Zhou, D. Li, et al., Adv. Mater. 28 (2016) 3516-3521. DOI:10.1002/adma.201504891 |
| [63] |
R. López, R. Gómez, J. Sol-Gel Sci. Technol. 61 (2012) 1-7. DOI:10.1007/s10971-011-2582-9 |
| [64] |
P. Raizada, A. Sudhaik, P. Singh, et al., J. Photochem. Photobiol. A:Chem. 374 (2019) 22-35. DOI:10.1016/j.jphotochem.2019.01.015 |
| [65] |
B.T. Huang, J.B. He, S.Y. Bian, et al., Chin. Chem. Lett. 29 (2018) 1698-1701. DOI:10.1016/j.cclet.2018.01.004 |
| [66] |
V.G. Reshma, P.V. Mohanan, J. Lumin. 205 (2019) 287-298. DOI:10.1016/j.jlumin.2018.09.015 |
| [67] |
M. Mahyari, Y. Bide, J.N. Gavgani, Appl. Catal. A 517 (2016) 100-109. DOI:10.1016/j.apcata.2016.03.010 |
| [68] |
Q. Zhang, W. Xu, C. Han, et al., Carbon 126 (2018) 128-134. DOI:10.1016/j.carbon.2017.10.006 |
| [69] |
X.L. Guo, Z.Y. Ding, S.M. Deng, et al., Carbon 134 (2018) 519-530. DOI:10.1016/j.carbon.2018.04.001 |
| [70] |
Liu Zhi, Wang Zhijian, Qing Shaojun, et al., Appl. Catal. B:Environ 232 (2018) 86-92. DOI:10.1016/j.apcatb.2018.03.045 |
| [71] |
M.T. Dejpasand, E.S. Iranizad, A. Bayat, A. Montaghemi, S.R. Ardekani, Mater. Res. Bull. 128 (2020) 110886. DOI:10.1016/j.materresbull.2020.110886 |
| [72] |
V. Ramar, S. Moothattu, K. Balasubramanian, Sol. Energy 169 (2018) 120-127. DOI:10.1016/j.solener.2018.04.040 |
| [73] |
V. Wongso, N.S. Sambudi, S. Sufian, Isnaeni, Biomass Convers. Biorefin. (2020), doi: http://dx.doi.org/10.1007/s13399-020-00662-9.
|
| [74] |
F.T. He, Y.X. Wang, J.Q. Zhang, et al., Appl. Surf. Sci. 495 (2019) 143558. DOI:10.1016/j.apsusc.2019.143558 |
| [75] |
J. Di, J.X. Xia, M.X. Ji, et al., ACS Sustain. Chem. Eng. 4 (2016) 136-146. DOI:10.1021/acssuschemeng.5b00862 |
| [76] |
G.L. Di, Z.L. Zhu, Q. Dai, et al., Chem. Eng. J. 379 (2020) 122296. DOI:10.1016/j.cej.2019.122296 |
| [77] |
Q.K. Chen, L. Chen, J.J. Qi, et al., Chin. Chem. Lett. 30 (2019) 1214-1218. DOI:10.1016/j.cclet.2019.03.002 |
| [78] |
Y.H. Zhang, Y.Y. Zhao, Z.L. Xu, et al., Appl. Catal. B: Environ. 262 (2020) 118306. DOI:10.1016/j.apcatb.2019.118306 |
| [79] |
W. Liu, Y.Y. Li, F.Y. Liu, et al., Water Res. 151 (2019) 8-19. DOI:10.1016/j.watres.2018.11.084 |
| [80] |
Y. Huang, Y.L. Liang, Y.F. Rao, et al., Environ. Sci. Technol. 51 (2017) 2924-2933. DOI:10.1021/acs.est.6b04460 |
| [81] |
F.L. Wang, P. Chen, Y.P. Feng, et al., Appl. Catal. B:Environ. 207 (2017) 103-113. DOI:10.1016/j.apcatb.2017.02.024 |
| [82] |
Chen Jibin, Che Huinan, Huang Kai, et al., Appl. Catal. B:Environ. 192 (2016) 134-144. DOI:10.1016/j.apcatb.2016.03.056 |
| [83] |
J.X. Xia, J. Di, H.T. Li, et al., Appl. Catal. B:Environ. 181 (2016) 260-269. DOI:10.1016/j.apcatb.2015.07.035 |
| [84] |
J.Z. Li, K. Liu, J.L. Xue, et al., J. Catal. 369 (2019) 450-461. DOI:10.1016/j.jcat.2018.11.026 |
| [85] |
H.Q. Xua, Y.H. Jiang, X.Y. Yang, et al., Mater. Res. Bull. 97 (2018) 158-168. DOI:10.1016/j.materresbull.2017.09.004 |
| [86] |
P. Chen, F.L. Wang, Z.F. Chen, et al., Appl. Catal. B:Environ. 204 (2017) 250-259. DOI:10.1016/j.apcatb.2016.11.040 |
| [87] |
R. Xie, L. Zhang, H. Xu, et al., Chem. Eng. J. 310 (2017) 79-90. DOI:10.1016/j.cej.2016.10.089 |
| [88] |
H. Wang, Z.Y. Wei, H. Matsui, S.Q. Zhou, J. Mater. Chem. A 2 (2014) 15740-15745. DOI:10.1039/C4TA03130J |
| [89] |
H.T. Li, X.D. He, Z.H. Kang, et al., Angew. Chem. Int. Ed. 122 (2010) 4532-4536. DOI:10.1002/ange.200906154 |
| [90] |
J.J. Wang, L. Tang, G.M. Zeng, et al., Appl. Catal. B:Environ. 222 (2018) 115-123. DOI:10.1016/j.apcatb.2017.10.014 |
| [91] |
R. Miao, Z. Luo, W. Zhong, et al., Appl. Catal. B:Environ. 189 (2016) 26-38. DOI:10.1016/j.apcatb.2016.01.070 |
| [92] |
R.R. Jiang, G.H. Lu, Z.H. Yan, et al., Chem. Eng. J. 374 (2019) 79-90. DOI:10.1016/j.cej.2019.05.176 |
| [93] |
S.T. Feng, T. Chen, Z.C. Liu, et al., Sci. Total Environ. 704 (2020) 135404. DOI:10.1016/j.scitotenv.2019.135404 |
| [94] |
M.X. Liang, Z.S. Zhang, R. Long, et al., Environ. Pollut. 259 (2020) 113770. DOI:10.1016/j.envpol.2019.113770 |
| [95] |
X.Z. Yuan, J. Zhang, M. Yan, et al., J. Colloid Interface Sci. 541 (2019) 123-132. DOI:10.1016/j.jcis.2019.01.072 |
| [96] |
J. Zhang, M. Yan, X.Z. Yuan, et al., J. Colloid Interface Sci. 529 (2018) 11-22. DOI:10.1016/j.jcis.2018.05.109 |
| [97] |
M.X. Ji, Y.L. Liu, J. Di, et al., Appl. Catal. B:Environ. 237 (2018) 1033-1043. DOI:10.1016/j.apcatb.2018.06.014 |
| [98] |
L.Y. Lin, S. Kavadiya, B.B. Karakocak, et al., Appl. Catal. B:Environ. 230 (2018) 36-48. DOI:10.1016/j.apcatb.2018.02.018 |
| [99] |
L. Xu, L. Yang, X. Bai, et al., Chem. Eng. J. 373 (2019) 238-250. DOI:10.1016/j.cej.2019.05.028 |
| [100] |
Z.G. Mou, H. Zhang, Z.M. Liu, et al., Appl. Surf. Sci. 496 (2019) 143655. DOI:10.1016/j.apsusc.2019.143655 |
| [101] |
S.J. Wang, I. Cole, Q. Li, Chem. Commun. 52 (2016) 9208-9211. DOI:10.1039/C6CC03302D |
| [102] |
R. Wang, K.Q. Lu, F. Zhang, et al., Appl. Catal. B:Environ. 233 (2018) 11-18. DOI:10.1016/j.apcatb.2018.03.108 |
| [103] |
J.J. Qian, C. Shen, J. Yan, et al., J. Phys. Chem. C 122 (2018) 349-358. DOI:10.1021/acs.jpcc.7b08702 |
| [104] |
M. Ebrahimi, M. Samadi, S. Yousefzadeh, et al., ACS Sustain. Chem. Eng. 5 (2017) 367-375. DOI:10.1021/acssuschemeng.6b01738 |
| [105] |
X.Y. Yue, X.L. Miao, Z.Y. Ji, et al., J. Colloid Interface Sci. 531 (2018) 473-482. DOI:10.1016/j.jcis.2018.07.086 |
 2020, Vol. 31
2020, Vol. 31