b Gordon Center for Medical Imaging, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02129, United States;
c State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing 210009, China
Indole derivatives are abundant in bioactive natural products [1, 2] and are known to exhibit important biological activities [3, 4]. Moreover, a huge number of natural and synthetic indoles have been applied to pharmaceuticals and agricultural chemicals [5]. Bis-indoles, in particular, have attracted much attention due to their biological activities including potent anticancer activity [6-8]. Such compounds are found to display remarkable antitumor activity with low toxicity in cancer cells by blocking the cell cycle progression [9]. Furthermore, bis(indolyl)methanes are also found in bioactive natural products such as streptindole, vibrindole A and arsindoline A [10] that possess anticancer [11-13], antibiotic [14], antibacterial and antiviral activities [15] (Fig. 1A). In addition, oxindoles are not only widely found in a variety of natural products such as uncarine F, isorhynchophylline, and isocorynoxeine [16] (Fig. 1B), but also exist in some drug candidates such as PLK4 inhibitor CFI-400945 [17] and MDM2 inhibitor SAR405838 [18] (Fig. 1). Oxindoles have been found to demonstrate anticancer [17, 19, 20], antimicrobial [21] and anticonvulsant activities [22]. Particularly, 3, 3-di(indolyl)indolin-2-ones have attracted intense attention due to the biologically relevant bis(indolyl)methane and oxindole moieties [23-28]. Numerous studies have revealed that 3, 3-di(indolyl)indolin-2-ones exhibit a wide variety of biological activities such as antimicrobial [29], anticancer [30] and spermicidal [31] activities.

|
Download:
|
| Fig. 1. Representative natural products (A and B) and drug candidates (C) containing the bis-indole (highlighted in blue) and oxindole (highlighted in red) ring, respectively | |
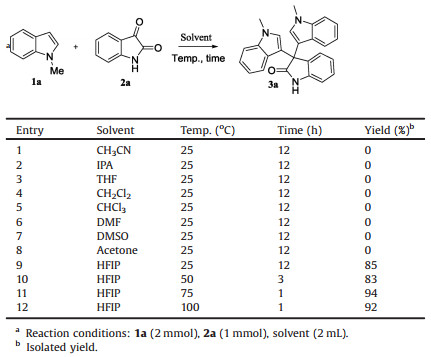
Because of unique structural features and interesting biological properties of 3, 3-di(indolyl)indolin-2-ones, the design of new strategies for the construction of this scaffold has attracted considerable interest. Consequently, numerous efforts have been devoted to the construction of it. Generally, 3, 3-di(indolyl)indolin-2-ones are prepared through the reactions of isatins with indoles [30]. Over the last few decades, a number of methods used silica sulfuric acid (SSA) [23], Amberlyst-15 [24], FeCl3 [25], ceric ammonium nitrate (CAN) [26], para-toluenesulfonic acid (TsOH) [27], heteropolyacid (H6P2W18O62) [28], silica-supported In(acac)3 [32], silicotungstic acid (H4SiW12O40) [33], I2 [31] and ionic liquids [34]. Although these methods have their own merits, they suffer from issues such as the use of expensive or toxic catalysts, long reaction time, harsh reaction condition, low yields and complicated operation. Therefore, development of efficient strategies is still highly desirable. In addition, hexafluoro-2-propanol (HFIP) is an extensively used green solvent in organic synthesis due to its unique properties such as strong H-bond donating (HBD) ability [35-38]. We previously reported that HFIP could promote C(sp2)-H heteroarylation [35] and nucleophilic addition reactions [36] that enabled efficient synthesis of biaryls and nonnatural α-arylated amino esters, respectively. Herein, we report the first HFIP-promoted catalyst-free efficient synthesis of biologically important 3, 3-di(indolyl)indolin-2-ones from indoles and isatins.
Initially, N-methylindole (1a, 2 mmol) and isatin (2a, 1 mmol) were chosen as model substrates to optimize the reaction conditions. Several solvents were examined, and the data are summarized in Table 1. The results revealed that HFIP was the optimal solvent for this reaction (Table 1, entries 9–12), other commonly used solvents such as CH3CN (acetonitrile), IPA (isopropanol), THF (tetrahydrofuran), CH2Cl2 (dichloromethane), etc., failed to give the corresponding product (Table 1, entries 1–8). To our delight, even at 25 ℃, HFIP could promote the reaction efficiently, affording compound 3a in 85% yield (Table 1, entry 9), indicating the effectiveness of HFIP. Increase of the temperature could improve the reactivity, giving compound 3a with increased yields and decreased reaction time. These results above highlight the importance of the temperature for the reactivity. For example, when the reaction was performed at 75 ℃, compound 3a was obtained in 94% yield (Table 1, entry 11), further increase of the temperature failed to improve the yield of the reaction, compound 3a was generated in 92% yield when the reaction was performed at 100 ℃. Therefore, the optimal condition was 1a (2 mmol), 2a (1 mmol), and HFIP (2 mL). It should be noted that no any additional catalyst was employed in this reaction, and equivalent amount of the starting materials were used. The reaction condition is mild and atom-economic, thus may have potential applications in organic synthesis.
|
|
Table 1 Optimization of the reaction condition. |
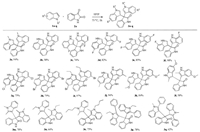
With the optimized reaction condition in hand, we next examined the scope of indole substrates. As shown in Scheme 1, various indoles reacted smoothly with isatin to give the corresponding 3, 3-di(indolyl)indolin-2-ones 3a-q in good to excellent yields (up to 98%) regardless of the position of substituents attached to the indole ring. We also noted that for N-unprotected indole and isatin substrates, the products were also obtained in good yields. For example, compound 3f was formed in 98% yield. Such compounds definitely allow further modifications on the NH group for late-state diversification, affording focused library for biological testing. The halo atom in indole substrates could also be utilized for diversification via the transition-metal catalysis. However, for indole substrates containing the electron-withdrawing group (e.g., nitro or cyano) and azaindoles, no corresponding products were obtained under the optimal conditions.
|
Download:
|
| Scheme 1. Substrate scope of indole substrates. | |
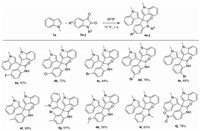
In view of the good reactivity of different indole substrates, we next examined the scope of isatin derivatives and found that various isatin derivatives also reacted smoothly with N-methylisatin to give the title compounds 4a-j in good yields (58%–93%), of which compound 4a was afforded in 93% yield (Scheme 2). Diverse functional groups in isatins such as fluoro, chloro, bromo, methyl, methoxyl and nitro were well tolerated. We found that the electron-withdrawing group (e.g., -NO2) attached to the oxindole ring had no remarkable effect on the reactivity, compound 4j was formed in 78% yield. Associated with above findings, we may conclude that the electron-density in the indole ring has a significant effect on the reactivity, while the electron-density in the oxindole ring has no effect on the reactivity. We speculate that the oxindole ring of the products could be cleaved under alkaline or acidic conditions to give the steric α, α, α-trisubstituted acetic acid derivatives, which are difficult to access through traditional synthetic methods.
|
Download:
|
| Scheme 2. Substrate scope of isatin substrates. | |
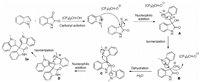
Finally, we proposed a plausible reaction mechanism for the synthesis of compound 3a (Scheme 3). Because of the strong H-bond donating ability of HFIP, the carbonyl group of isatin is activated by HFIP, followed by addition of N-methylindole, giving the unstable intermediate A. Isomerization of A gives the 3-indolyl-3-hydroxyl oxindole B, which is then activated by HFIP to give the intermediate C. Further nucleophilic addition of indole to C forms the intermediate D, which is then subjected to isomerization, forming compound 3a. It is anticipated that the biologically important 3-indolyl-3-hydroxyl oxindole could be formed if one equivalent of indole is used in this reaction. The reaction mechanism may explain the observed effects of the electron density of the indole and isatin ring on the reactivity. Different from previously reported protocols, no acidic catalyst is needed for this reaction, further suggesting the strong H-bond donating ability of HFIP.
|
Download:
|
| Scheme 3. Proposed mechanism. | |
In summary, we have developed an efficient, HFIP-promoted, and catalyst-free protocol for the synthesis of biologically relevant 3, 3-di(indolyl)indolin-2-ones (27 examples) from readily available indoles and isatin derivatives. This protocol shows well tolerance of different functional groups and thus enables the rapid access to various 3, 3-di(indolyl)indolin-2-ones in good to excellent yields (up to 98% yield). Compared with previously reported methods, this protocol has several merits such as short reaction time (~ 1 h), no usage of catalyst, easy operation and product isolation. Of note, there are two C—C bonds and one allcarbon quaternary center formed simultaneously in this process. This protocol could serve as an alternative strategy to synthesize biologically important 3, 3-di(indolyl)indolin-2-ones for biological testing. The functional groups in the indole and oxindole ring including the amide group could be utilized for late-state modification.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 81773562, 81703326 and 81973177); The Open Project of State Key Laboratory of Natural Medicines (No. SKLNMKF202005); China Postdoctoral Science Foundation (Nos. 2018M630840 and 2019T120641).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2020.03.025.
| [1] |
I. Ninomiya, J. Nat. Prod. 55 (1992) 541-564. DOI:10.1021/np50083a001 |
| [2] |
S. Yuan, D.Q. Zhang, J.Y. Zhang, et al., Org. Lett. 22 (2020) 814-817. DOI:10.1021/acs.orglett.9b04241 |
| [3] |
E. Huang, L. Zhang, C. Xiao, et al., Chin. Chem. Lett. 30 (2019) 2157-2159. DOI:10.1016/j.cclet.2019.04.044 |
| [4] |
L. Meng, Q. Guo, M. Chen, et al., Chin. Chem. Lett. 29 (2018) 1257-1260. DOI:10.1016/j.cclet.2017.12.001 |
| [5] |
M. Shiri, Chem. Rev. 112 (2012) 3508-3549. DOI:10.1021/cr2003954 |
| [6] |
A. Andreani, S. Burnelli, M. Granaiola, et al., J. Med. Chem. 51 (2008) 4563-4570. DOI:10.1021/jm800194k |
| [7] |
W.R. Chao, D. Yean, K. Amin, et al., J. Med. Chem. 50 (2007) 3412-3415. DOI:10.1021/jm070040e |
| [8] |
G. Giannini, M. Marzi, M.D. Marzo, et al., Bioorg. Med. Chem. Lett. 19 (2009) 2840-2843. DOI:10.1016/j.bmcl.2009.03.101 |
| [9] |
T. Andey, A. Patel, T. Jackson, et al., Eur. J. Pharm. Sci. 50 (2013) 227-241. DOI:10.1016/j.ejps.2013.07.007 |
| [10] |
G. Wang, J. Wang, Z. Xie, et al., Bioorg. Chem. 72 (2017) 228-233. DOI:10.1016/j.bioorg.2017.05.006 |
| [11] |
T. Inamoto, S. Papineni, S. Chintharlapalli, et al., Mol. Cancer Ther. 7 (2008) 3825-3833. DOI:10.1158/1535-7163.MCT-08-0730 |
| [12] |
Nachshon-Kedmi M., S. Yannai, F.A. Fares, Br. J. Cancer 91 (2004) 1358-1363. DOI:10.1038/sj.bjc.6602145 |
| [13] |
N. Ichite, M.B. Chougule, T. Jackson, et al., Clin. Cancer Res. 15 (2009) 543-552. DOI:10.1158/1078-0432.CCR-08-1558 |
| [14] |
M. KOBAYASHI, S.J. AOKI, K. GATO, et al., Chem. Pharm. Bull. 42 (1994) 2449-2451. DOI:10.1248/cpb.42.2449 |
| [15] |
P. Praveen, P. Parameswaran, M. Majik, Synthesis 47 (2015) 1827-1837. DOI:10.1055/s-0034-1380415 |
| [16] |
G. Ziarani, P. Gholamzadeh, N. Lashgari, et al., ARKIVOC i (2013) 470-535. |
| [17] |
B. Yu, Z. Yu, P.P. Qi, et al., Eur. J. Med. Chem. 95 (2015) 35-40. DOI:10.1016/j.ejmech.2015.03.020 |
| [18] |
S.M. Wang, W. Sun, Y.J. Zhao, et al., Cancer Res. 74 (2014) 5855-5865. DOI:10.1158/0008-5472.CAN-14-0799 |
| [19] |
B. Yu, D.Q. Yu, H.M. Liu, Eur. J. Med. Chem. 97 (2015) 673-698. DOI:10.1016/j.ejmech.2014.06.056 |
| [20] |
B. Yu, Y.C. Zheng, X.J. Shi, et al., Anticancer Agents Med. Chem. 16 (2016) 1315-1324. DOI:10.2174/1871520615666151102093825 |
| [21] |
Y.T. Yang, J.F. Zhu, G. Liao, et al., Curr. Med. Chem. 25 (2018) 2233-2244. DOI:10.2174/0929867325666171129131311 |
| [22] |
F.D. Popp, H. Pajouhesh, J. Pharm. Sci. 71 (1982) 1052-1054. DOI:10.1002/jps.2600710924 |
| [23] |
J. Azizian, A.A. Mohammadi, N. Karimi, et al., Cat. Commun. 7 (2006) 752-755. DOI:10.1016/j.catcom.2006.01.026 |
| [24] |
Y. Sarrafi, K. Alimohammadi, M. Sadatshahabi, et al., Monatsh. Chem. 143 (2012) 1519-1522. DOI:10.1007/s00706-012-0723-7 |
| [25] |
A. Kamal, Y.V. Srikanth, M.N. Khan, et al., Bioorg. Med. Chem. Lett. 20 (2010) 5229-5231. DOI:10.1016/j.bmcl.2010.06.152 |
| [26] |
S.Y. Wang, S.J. Ji, Tetrahedron 62 (2006) 1527-1535. DOI:10.1016/j.tet.2005.11.011 |
| [27] |
W. Huang, L. Nang, X. Li, et al., Chin. J. Chem. 33 (2015) 1167-1172. DOI:10.1002/cjoc.201500374 |
| [28] |
K. Alimohammadi, Y. Sarrafi, M. Tajbakhsh, Monatsh. Chem. 139 (2008) 1037-1039. DOI:10.1007/s00706-008-0885-5 |
| [29] |
A.R. Karimi, Z. Dalirnasab, G.H. Yousefi, et al., Res. Chem. Intermediat. 41 (2015) 10007-10016. DOI:10.1007/s11164-015-2007-4 |
| [30] |
Subba Reddy B.V., N. Rajeswari, M. Sarangapani, et al., Bioorg. Med. Chem. Lett. 22 (2012) 2460-2463. DOI:10.1016/j.bmcl.2012.02.011 |
| [31] |
P. Paira, A. Hazra, S. Kumar, et al., Bioorg. Med. Chem. Lett. 19 (2009) 4786-4789. DOI:10.1016/j.bmcl.2009.06.049 |
| [32] |
R.K. Sharma, C. Sharma, J. Mol. Catal. A Chem. 332 (2010) 53-58. DOI:10.1016/j.molcata.2010.08.020 |
| [33] |
K. Nikoofar, Arab J. Chem. 10 (2017) 283-287. DOI:10.1016/j.arabjc.2014.07.008 |
| [34] |
Rad-Moghadam K., Sharifi-Kiasaraie M., Taheri-Amlashi H., Tetrahedron 66 (2010) 2316-2321. DOI:10.1016/j.tet.2010.02.017 |
| [35] |
S. Yuan, B. Yu, H.M. Liu, Adv. Synth. Catal. 361 (2019) 59-66. DOI:10.1002/adsc.201801226 |
| [36] |
Z. Li, B. Yu, Chem. Eur. J. 25 (2019) 16528-16532. DOI:10.1002/chem.201904395 |
| [37] |
I.A. Shuklov, N.V. Dubrovina, A. Börner, Synthesis 2007 (2007) 2925-2943. DOI:10.1055/s-2007-983902 |
| [38] |
R.H. Vekariya, J. Aube, Org. Lett. 18 (2016) 3534-3537. DOI:10.1021/acs.orglett.6b01460 |
 2020, Vol. 31
2020, Vol. 31