b University of Chinese Academy of Sciences, Beijing 100049, China;
c School of Environment, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China
Tracking reaction processes on the surface of nanocrystals (NCs) in situ is a key issue for understanding the catalytic mechanisms and reaction kinetics [1]. As a promising fingerprint technique, surface-enhanced Raman spectroscopy (SERS) with greater sensitivity down to single molecules allows revealing surface catalytic processes with fast response and surface selectivity [2, 3]. To this end, great efforts have been devoted to fabrication of desired bifunctional platform which possess both of the high SERS and catalytic activity [4, 5]. However, integrating both SERS and catalytic activity into small-sized NCs is still of great challenge, let alone the small-sized alloy nanostructures. This is because both of alloy and small size nanostructures are detrimental to SERS applications [6], but favorable to catalytical performance [7].
From the structural point of view, core/shell NCs with highly-branched alloy shell have the ideal structure to achieve this goal. Firstly, the highly-branched shell with rough surface and core/shell structure can offer high SERS activity [2, 8], meanwhile the abundant atomic steps, edges, and corner atoms in the branches can boost their catalytical performance [9]. Both of alloy and core/shell structures can markedly enhance the stability and prevent agglomeration [10], which is of great importance for in situ monitoring reaction in aqueous dispersion. Further, the morphology and size distribution of core/shell NCs can be easily controlled through the seed-mediated-growth method. Considerable research efforts have been devoted to synthesis of multi-metallic branched NCs with alloy or core/shell structures [11, 12]. However, few studies devoted to synthesis of highly branched NCs with both alloy and core/shell structures and their application in in situ monitor catalytical reaction.
Herein, we report the facile synthesis of flower-like Au@AgPd trimetallic nanostructure (Au@AgPd NFs), through simultaneous selective growth of Ag and Pd on Au core for forming highly branched and highly dispersed AgPd alloy shell. The as-synthesized Au@AgPd NFs were highly monodispersed and superior stability in H2O2 solution. Most importantly, this small-sized Au@AgPd NFs showed excellent SERS and catalytic activity, and was successfully applied to in situ monitor catalytic reaction process. The exploration of such ideal nanostructures and the involved growth mechanisms are of great fundamental interest. The role of capping agent, Ag/Pd ratio, and core sizes were systematically studied. This study provides a vivid example to rational design versatile NCs by means of heterogeneous construction for fabrication of SERS platform, and may offer inspiration for tailoring other metal NCs to monitor different reactions with the understanding of involved growth mechanisms.
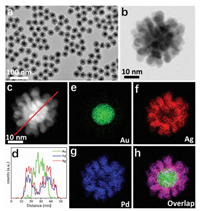
Seed-mediated growth method enables the controlled synthesis of monodispersed core/shell nanostructures owing to their manageable sizes and morphologies, and was thus employed to construct the highly-branched nanostructures. Gold nanocrystals (AuNCs) as the commonly used seeds for epitaxial growth endow shell with enhanced catalytic activity and boosted stability in aqueous dispersions. More importantly, the crystallographic defects in the twinned Au seed particles facilitates the growth of AgPd branches [13]. Therefore, the fine Au seeds were firstly synthesized with a narrow size distribution of 13.5±1.0 nm (Fig. S1 in Supporting information). Utilizing these AuNCs as seeds for simultaneous overgrowth of palladium and silver enables the formation of core/shell structured Au@AgPd NFs with highly-branched AgPd alloy shell, a narrow size distribution, enhanced stability, and high yield. In brief, AgNO3 and H2PdCl4 were coreduced by L-ascorbic acid (AA) and overgrown on Au seeds using CTAB as the capping agent. Experimental details can be found in Supporting information. To ensure a highly-dispersed alloy shell, the kinetics of Pd growth was controlled using a syringe pump, and the nucleation and growth processes will be discussed vide infra. Fig. 1 shows transmission electron microscopy (TEM) and scanning transmission electron microscopy (STEM) of the as-prepared Au@AgPd NFs, which is highly-branched with distinct core/shell structure. The Au@AgPd NFs show a narrow size distribution of diameter (38.4±2.1 nm), as well as branch length (10.5±1.3 nm) and width (5.4±0.7 nm) as shown in Fig. S2a (Supporting information). The inductively coupled plasma mass spectrometer (ICP-MS) analysis showed the overall weight percentages of Au, Ag and Pd in the Au@AgPd NFs were respectively 14.2%, 40.6% and 45.3%, which agreed with the ratio of Au/Ag/Pd precursors. The high yield of Au@AgPd NFs (90.0%, based on the total amount of Ag and Pd) indicates most of AgNO3 and H2PdCl4 were reduced and grew on AuNCs.
|
Download:
|
| Fig. 1. Morphology characterization of Au@AgPd NCs. (a) Low-magnification TEM image, (b) high-magnification STEM image, (c) HAADF-STEM image, (e-h) EDX elemental mappings and EDX line profile. | |
To explore the shell structure, the wide-angle X-ray diffraction (XRD) pattern was collected (Fig. S3 in Supporting information). Although the peaks of Ag and Au cannot be resolved due to their small lattice mismatch (0.9%), the presence of two characteristic diffraction peaks confirmed the alloy composition. Furthermore, X-ray photoelectron spectroscopy (XPS) was adopted to analyze the surface composition and state of Au@AgPd NFs within 2 nm, and results showed that both Ag and Pd existed in the surface of Au@AgPd NFs (Fig. S4 in Supporting information). The binding energy of Pd 3d is evidently shifted to 335.7 and 340.9 eV compared to the standard Pd 3d peaks (335.2 and 340.4 eV), which suggests that there is electron transfer from Pd to Ag due to the alloying of Ag and Pd. To directly observe the spatial distributions of Ag, Pd and Au in Au@AgPd NFs, high-angle annular darkfield TEM (HAADF-TEM) equipped with energy-dispersive X-ray (EDX) elemental mapping was employed, and results are shown in Fig. 1. By overlaying these elemental maps, it can be seen that Au element clearly separated from Ag and Pd elements, confirming the core/shell structure; while the well overlapped flower-like Ag and Pd maps indicated the alloy structure of AgPd branch. The crosssectional composition line profiles also suggest the homogeneity of the AgPd shell. Taken together, the highly-branched shell are demonstrated alloy structure in full-scale. The stability of this alloyed shell was demonstrated by unchanged morphology of Au@AgPd NFs after reaction with H2O2 (2 wt%) for 20 min (Fig. S5 in Supporting information). This result also implied that the alloy structure can protect Ag from etching. In addition, the Au@AgPd NFs showed excellent SERS activity in comparison with Au@Pd NCs (Fig. S6 in Supporting information). It should note that the Au@Pd NCs tightly coated with Pd shell also showed slight SERS activity, which suggest the core/shell structure endowed Pd shell with SERS activity. The result of finite difference time domain (FDTD) simulation in Fig. S7 (Supporting information) further confirmed this conclusion. The reason for markedly enhanced SERS activity of Au@AgPd NFs may be multiple. Highly-branched shell, addition of Ag, core/alloy-shell structure may endow the small-sized Au@AgPd NFs with SERS activity together. However, reliable and accurately quantitative statements on the SERS enhancement factor of this complex nanostructure are difficult to be achieved due to the unknown parameters of molar extinction coefficient and exact surface area of the Au@AgPd NFs [4].
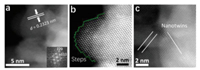
To further investigate the structure of AgPd branches down to the atomic level, aberration-corrected HAADF-STEM imaging was conducted. The lattice fringes were clearly observed when the Au@AgPd nanoparticle is viewed along the [110] zone axis, indicating the good crystallization (Fig. 2a). Although discrimination of Ag and Pd atoms through Z-contrast difference is impossible due to their adjacent atomic number, AgPd alloy can be confirmed in atomic level by measuring their lattice spacings. As shown in Fig. 2a, the average lattice spacing of AgPd branch is determined as 0.2323 nm, which is between that of the (111) plane of Ag (0.2370 nm) and Pd (0.2230 nm), confirming the AgPd alloy structure. It is noteworthy that in the AgPd alloy branch there are abundant lattice defects including steps, atomic corners and nanotwins (Figs. 2b and c), which could act as highly efficient catalytic sites.
|
Download:
|
| Fig. 2. (a) Aberration-corrected HAADF-STEM image of branches in Au@AgPd NCs. Step (b) and nanotwins (c) in branches. The inset image in (a) shows the fast Fourier transform (FFT) pattern. | |
To elucidate the mechanism involved in the formation of highly-branched Au@AgPd NFs, a set of experiments were conducted. The role of CTAB was firstly investigated because it has been widely used in morphological regulation [14-16]. With no addition of CTAB, the synthesized Au@AgPd NFs showed rough surface and wide branches (Fig. S8 in Supporting information). This result suggests that CTAB may not be the key factor governing the branch formation, but can assist AgPd bloom on Au seeds forming a highly-branched shell. The possible explanation is that the Ag and/or Pd nucleated on AuNPs surface and formed Ag and/or Pd islands, then CTAB capped on the surface of the islands [17] with lower density on the island top than that on the sides due to their difference in curvature [18]. This resulted in the deposition of Ag and Pd atoms on the island top rather than its sides, therefore, the formation of narrowed AgPd branches. In the absence of CTAB, however, the difference of AgPd deposition rate between island top and sides is not that large, and hence broad branches formed.
The molar ratio of Pd to Ag was found to markedly affect the shell growth. As illustrated in Fig. 3, core/shell structured Au@Pd or Au@Ag NCs with smooth and rather highly-branched shell was formed when H2PdCl4 or AgNO3 was used as precursor alone. However, distinct branches appeared when co-reducing both Pd and Ag precursors with various Pd/Ag ratios, which imply the critical role of the coexistence of Ag and Pd metals in the branch formation. Specifically, the branches changed from sparse to compact and then to sparse again with increasing of Pd/Ag ratio, and the Pd/Ag ratio of 1:1 is the optimal point at which closely spaced, and uniform branches were obtained. This was attributed to the large lattice mismatch (6.3%), as metals tend to deposit on substrate in the manner of island growth (Volmer-Weber mode) when lattice mismatch larger than 5% [19].

|
Download:
|
| Fig. 3. Morphologies of Au@AgPd NCs with various Pd/Ag ratio of (a) 0:10, (b) 2:10, (c) 5:10, (d) 10:10, (e) 20:10 and (f) 10:0. | |
The formation of highly-branched shell is also dependent on the size of Au seed. Citrate-coated AuNCs with different sizes were at first prepared (Table S1 and Fig. S1 in Supporting information) to study the branch shell. As shown in Fig. 4, large sized Au core inhibited the formation of closely compact branch shell. When the diameter of Au seeds increased to 60 nm, branches can hardly be observed. Furthermore, the branches tend to grow at the corner of Au seeds (Fig. 4c), by which the high surface energies of corner can be released and thus a more stable structure is adopted. Consistently, smaller sized Au seeds have more twin boundary sites facilitating the AgPd nucleation, and thus densely branches tend to grow on small sized AuNPs. Additionally, it should be noted that the sodium citrate (SC) concentration in all growth solutions with different AuNCs sizes were kept closely in the range of 0.13–0.14 mmol/L (Fig. S9 in Supporting information), which suggested the impact of SC on branches growth is not remarkable.

|
Download:
|
| Fig. 4. Morphologies of Au@AgPd NFs (down) with the corresponding core diameter (up) under the proposed synthesis conditions. | |
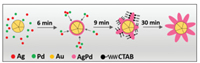
To further elucidate the formation mechanism of this highly-branched shell, the shape-evolution process of the Au@AgPd NFs was studied. It should be mentioned that the growth of Ag was slow due to the retarded kinetics in the presence of CTAB [19], and the Ag reduction relies mostly on galvanic replacement between Ag and Pd [20], i.e., the Ag growth kinetics is dependent on the amount of Pd. Thus, the growth kinetics of both Ag and Pd can be controlled by slowly injecting H2PdCl4 with a syringe pump, which provides enough time to prepare TEM samples to track the Au@AgPd NFs evolution. Fig. S10 (Supporting information) shows the TEM images of aliquots sampled from reaction solution at different reaction times. After 3 min from injecting AgNO3, only multiply twinned Au NCs are shown in the TEM images (Fig. S10a). Then, a smooth and thin shell on Au seed was observed after reaction for 6 min (Fig. S10b). After 9 min, branches were appeared at the twin boundary sites which is consistent with above conclusion (Fig. S10c). Further, branched shell was formed at 12 min (Fig. S10d) and become more densely at 15 min (Fig. S10e). With the extension of reaction time to 30 min, no apparent changes in morphology were further detected (Fig. S10f).
Collectively, the growth mechanism of high-branched shell can be concluded as illustrated in Fig. 5. Firstly, a thin film of Ag and/or Pd was formed on Au seed. Then, Ag and Pd were selectively codeposited on the twin boundary sites of Au NCs forming small islands. After that, Ag and Pd deposit on the top of the islands with the assistance of CTAB to form branches. Finally, uniform and dense branches are formed on Au NCs surface with continuing growth.
|
Download:
|
| Fig. 5. Schematic diagram of the branch growth process. | |
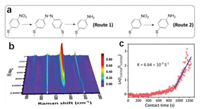
Given the highly-branched Au@AgPd NFs possessed abundant defects and rough surface that are favored to both catalysis and SERS applications, it was applied to in situ SERS monitor the model reaction of Pd-catalyzed hydrogenation of 4-nitrothiophenol (4-NTP) to 4-aminothiophenol (4-ATP). To acquire stable and highresolution temporal SERS spectra, Au@AgPd NFs were deposited on a quartz plate and dried at room temperature. Specifically, a monolayer of Au@AgPd NFs was assembled at the air/liquid interface at first. Then, the assembled monolayer was transferred onto a hydroxylated quartz plate for fabricating a SERS platform with uniform hotspots. With the prepared platform, the hydrogenation of 4-NTP was in situ recorded and their reaction kinetics were achieved as shown in Fig. 6 (for more experimental details see Supporting information). As shown in the in situ SERS spectra (Fig. 6b), initially on the AgPd branches were pre-adsorbed with a monolayer of 4-NTP molecules with four characteristic bands located at 854, 1076, 1337, and 1572 cm-1, which are assigned to C—H wagging (υCH), C—S stretching (υCS), O—N—O stretching (υNO2), and C—C stretching (υCC), respectively [5]. The intensities of R-NO2-related bands decrease as the reaction proceeds, and concomitantly new bands of 1593 cm-1, corresponding to C—C stretching (υCC), gradually appeared. This change indicates that 4-NTP was efficiently reduced to 4-ATP on the alloy branches.
|
Download:
|
| Fig. 6. In situ SERS monitoring of the catalytic reduction of 4-NTP to 4-ATP. (a) Reaction routes of 4-NTP reduction on the catalytic surface. (b) In situ SERS spectra. (c) Plots of the logarithm of the corresponding SERS intensity at 1337 cm-1 (ln(I1337(t)/I1337(0)) versus the reaction time. | |
The SERS measurement also enabled the elucidation of in detailed reaction mechanisms. Previous studies [21-23] showed that two reaction routes schemed in Fig. 6a are involved in the 4-NTP reduction: (1) The molecules are adsorbed on the catalyst, then dimerized into 4, 4'-dimercaptoazobenzene (DMAB) with adjacent 4-NTP, and further reduced into 4-ATP (Route 1); (2) hydrogen dissociated on the catalyst surface and then directly reduce the 4-NTP molecules into 4-ATP (Route 2). The characteristic bands of the intermediate located at 1140, 1390 and 1435 cm-1 were not detected, suggesting Route 2 dominate the reduction process [22, 23]. This is because the Pd NCs are highly active for hydrogen dissociation and thus facilitate the Route 2 reaction.
The reaction kinetics was further measured by quantitatively monitoring the intensity decrease of the characteristic bands at 1337 cm-1. Because no DMAB intermediates involved in the reduction reaction as analysed above, and the large excess of NaBH4, the reduction reaction can be assumed to follow pseudo-first-order kinetics. The reaction rate constant (k) can be calculated by Eq. (1):
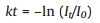
|
(1) |
where t is the reaction time, and I is the intensity of the band at 1337 cm-1. As plotted in Fig. 6c, the reaction rate is slow in the initial 900 s, suggesting the requirement of a period of induction time. Although further studies are required to clarify this induction period, a possible tentative explanation is the fact that both of diffusion process of BH4– to the catalysis center and activation process are time-consuming. Besides, the duration time of the induction period is closely related to laser power, but the reaction rate constant (k) is not influenced based on our previous study [24]. Thus, the first-ordered fit was conducted in the range of 900–1200s, and a linear correlation between ln(It/I0) and reaction time (t) was found as shown in Fig. 6c with reaction rate constant of 6.64×10-3 s-1.
In summary, both catalytic and SERS activity were integrated into the small-sized Au@AgPd NFs through a facile seed-mediatedgrowth method. The as-synthesized Au@AgPd NFs shows many advantages, such as small sizes, highly-dispersed alloy shell, and defect abundance that were favorable to catalytic process but detrimental to its SERS activity; while the advantages of monodispersion, highly dense branches, core/alloy-shell structures settled this dilemma. The growth mechanism of this fascinating nanostructure was thus explored. The Pd/Ag ratio and twin boundary sites were shown to be crucial for the formation of branched shell, while CTAB assists AgPd to bloom on Au seed with narrowed branches. Owing to their SERS and catalytic activity, the Au@AgPd NFs were successfully applied to fabricating bifunctional SERS platform which was demonstrated enable to in situ monitor the catalytic conversion of 4-NTP to 4-ATP. This study provides a vivid example to construct bifunctional nanocrystals with small sizes and alloy structure to in situ monitor catalytic reaction, and this may offer inspiration for tailoring other metal NCs to monitor different reactions with the understanding of involved growth mechanisms.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Key R&D Program of China (No. 2016YFA0203102), and the National Natural Science Foundation of China (Nos. 21620102008 and 21827815).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.050.
| [1] |
S. Liu, Y. Ying, Y. Long, Chin. Chem. Lett. 31 (2020) 473-475. DOI:10.1016/j.cclet.2019.07.057 |
| [2] |
J.F. Li, Y.F. Huang, Y. Ding, et al., Nature 464 (2010) 392-395. DOI:10.1038/nature08907 |
| [3] |
C. Huang, F. Lu, K. Xu, et al., Chin. Chem. Lett. 30 (2019) 2009-2012. DOI:10.1016/j.cclet.2019.02.006 |
| [4] |
W. Xie, C. Herrmann, K. Kompe, M. Haase, S. Schlucker, J. Am. Chem. Soc. 133 (2011) 19302-19305. DOI:10.1021/ja208298q |
| [5] |
J. Huang, Y. Zhu, M. Lin, et al., J. Am. Chem. Soc. 135 (2013) 8552-8561. DOI:10.1021/ja4004602 |
| [6] |
X. Tang, W. Cai, L. Yang, J. Liu, Nanoscale 6 (2014) 8612-8616. DOI:10.1039/C4NR01939C |
| [7] |
A.M.S. Pembere, C. Cui, H. Wu, Z. Luo, Chin. Chem. Lett. 30 (2019) 1000-1004. DOI:10.1016/j.cclet.2018.12.019 |
| [8] |
Y. Lu, C.Y. Zhang, D.J. Zhang, et al., Chin. Chem. Lett. 27 (2016) 689-692. DOI:10.1016/j.cclet.2016.01.032 |
| [9] |
C. Li, Q. Yuan, B. Ni, et al., Nat. Commun. 9 (2018) 3702. DOI:10.1038/s41467-018-06043-1 |
| [10] |
R. Ghosh Chaudhuri, S. Paria, Chem. Rev. 112 (2012) 2373-2433. DOI:10.1021/cr100449n |
| [11] |
Y. Feng, H. Liu, J. Yang, J. Mater. Chem. A 2 (2014) 6130-6137. DOI:10.1039/C3TA14121G |
| [12] |
J. Yang, L. Xia, Z. Lin, et al., Chin. Chem. Lett. 30 (2019) 638-642. DOI:10.1016/j.cclet.2018.08.004 |
| [13] |
L. Zhou, Z. Liu, H. Zhang, et al., Nanoscale 6 (2014) 12971-12980. DOI:10.1039/C4NR04190A |
| [14] |
C.W. Yang, K. Chanda, P.H. Lin, et al., J. Am. Chem. Soc. 133 (2011) 19993-20000. DOI:10.1021/ja209121x |
| [15] |
C. Zhu, H.C. Peng, J. Zeng, et al., J. Am. Chem. Soc. 134 (2012) 20234-20237. DOI:10.1021/ja3091214 |
| [16] |
D.C. Niu, Z. Ma, Y.S. Li, J.L. Shi, J. Am. Chem. Soc. 132 (2010) 15144-15147. DOI:10.1021/ja1070653 |
| [17] |
B. Nikoobakht, M.A. El-Sayed, Chem. Mater. 15 (2003) 1957-1962. DOI:10.1021/cm020732l |
| [18] |
H. Hinterwirth, S. Kappel, T. Waitz, et al., ACS Nano 7 (2013) 1129-1136. DOI:10.1021/nn306024a |
| [19] |
F.R. Fan, D.Y. Liu, Y.F. Wu, et al., J. Am. Chem. Soc. 130 (2008) 6949-6951. DOI:10.1021/ja801566d |
| [20] |
H. Zhang, Z. Liu, X. Kang, et al., Nanoscale 8 (2016) 2242-2248. DOI:10.1039/C5NR07333B |
| [21] |
H. Zhang, X.G. Zhang, J. Wei, et al., J. Am. Chem. Soc. 139 (2017) 10339-10346. DOI:10.1021/jacs.7b04011 |
| [22] |
J. Zhang, S.A. Winget, Y. Wu, et al., ACS Nano 10 (2016) 2607-2616. DOI:10.1021/acsnano.5b07665 |
| [23] |
Q. Han, C. Zhang, W. Gao, et al., Sens. Actuators B-Chem. 231 (2016) 609-614. DOI:10.1016/j.snb.2016.03.068 |
| [24] |
R. Liu, Z.L. He, J.F. Sun, J. Liu, G.B. Jiang, Small 12 (2016) 6378-6387. DOI:10.1002/smll.201601773 |
 2020, Vol. 31
2020, Vol. 31 

