b Guangdong Provincial Key Laboratory of Radionuclides Pollution Control and Resources, Guangzhou University, Guangzhou 510006, China
Picric acid (PA), IUPAC named 2, 4, 6-trinitrophenol, is one of the primary explosive ingredients with stronger explosion power than the well-known explosive 2, 4, 6-trinitrotoluene (TNT) [1, 2]. It is used extensively for rocket fuels manufacturing, dye and pharmaceuticals industries [3]. Besides being a serious threat to the environmental and public safety, PA is also considered to be a food safety risk factor owing to its high solubility in water and difficulty to be degraded [4, 5]. Long exposure to PA could lead to diseases of human respiratory system and liver damage through the food chain transmission and enrichment [6]. Therefore, increasing attentions have been put on the sensing of PA with high performance for the concerns of homeland security, public and food safety.
Numerous analytical techniques were established for the detection of PA including liquid chromatography-high resolution accurate mass spectrometry [7], surface-enhanced Raman spectrometry [8], energy dispersive X-ray diffraction [9], electrochemical methods [10], capillary electrophoresis [11], ion mobility spectrometry [12], and so forth. Although distinct advantages have been shared between these methods, some drawbacks such as the requirement of expensive instruments, time-consuming sample preparation and high test cost, still existed. Hence, developing simple, rapid and visual sensors for PA assay remain in high demand. Optical probes based on synthetic receptors and nanomaterials provide effective approaches for the visual detection of many kinds of analytes because of their ease of observation, low cost, high sensitivity and specificity [13-18]. To date, many of them have been applied in the sensing of PA by taking these advantages [19-24]. However, most of the present approaches rely on the luminescence quenching of probe induced by PA, whereas the colorimetric detecting modes are very rare [1, 25]. Not only that, many issues in this process remain unsolved yet, for example, laborious synthesis, the need of organic solvent originated from their poor water solubility, emission or excitation in the invisible region [26, 27].
Water-soluble poly(3-alkoxy-4-methylthiophene)s (P3RO-4MeTs) are one special kind of conjugated polymers (CPs) which feature efficient signal amplification and excellent biocompatibility [28, 29]. Compared with other CPs, P3RO-4MeTs have their own merits including ease of functionalization, fast response and sensitive conformation to external stimuli [30, 31]. In this paper, we employ ionic self-assembly of P3RO-4MeT-based probes to construct a sensing system with colorimetric and fluorometric dual responses, allowing for visual detection and real-time monitoring of PA. For this purpose, a novel P3RO-4MeTs derivative, poly(3-(4-methyl-3'-thienyloxy) propyldimethyl benzyl ammonium chloride) (PMTPBA, Scheme 1) was designed and synthesized. In this probe, the polymer backbone is modified with a benzyl ammonium moiety to enhance the non-covalent interactions between the probe and PA. This group not only endows PMTPBA excellent water solubility, but also promotes electrostatic, charge transfer and π-π stacking interactions between PA and PMTPBA itself. Meanwhile, we expect the steric effect of phenyl group could effectively keep the luminescence of PMTPBA by preventing the self-aggregation of polythiophene in solid support. Based on the above considerations, we anticipate that supramolecular aggregates of PMTPBA would be formed in the presence of PA, thereby resulting in colorimetric and fluorescent dual signal outputs (Scheme 1). Thus, the proposed approach would be developed as a much effective method for PA visual detection in 100% aqueous media and fabricated to test strip in solid state. As far as we know, this is the first time that a water soluble probe has been developed for fluorometric and colorimetric dual detection of PA based on the formation of supramolecular assemblies. This mechanism is very different from the methods previously reported whose color changes mainly originated from PA itself [32]. To validate the proposed strategy, the details and experimental results will be discussed in the subsequent sections.

|
Download:
|
| Scheme 1. Chemical structures of PMTPBA, PA and schematic illustration of the proposed sensing mechanism. | |
Water-soluble PMTPBA was prepared by treating 3-(3-bromopropoxy)-4-methylthiophene with N, N-dimethylbenzylamine and then polymerization utilizing FeCl3 as the oxidizing agent in chloroform following the previously reported procedure (Figs. S1–S9 in Supporting information) [30]. It exhibited excellent water solubility and a yellow color in aqueous solution. The optical behaviors of PMTPBA in the absence and presence of various amounts of PA was first examined by absorption spectra (Fig. 1A). It was found that the absorption peak of PMTPBA alone in HEPES buffer (10 mmol/L, pH 7.4) appeared at 410 nm, which indicated that the polymer probe was well dispersed in solution and the backbone was adopted with a random coil conformation [31]. Upon addition of increasing amounts of PA, the absorbance of PMTPBA in the range of 500–600 nm gradually increased and accompanied by the solution color changing from yellow to dark orange, indicating a transition from random-coil to more ordered phase of the polymer backbone. Fig. 1B showed the relative absorption intensity at 410 nm and 540 nm of PMTPBA in pace with titrating with PA in HEPES buffer. It had been found that there was a good linear relationship between the ratio of A540/A410 and the concentration of PA (R2 = 0.997, from 0.1 μmol/L to 15 μmol/L). These results signified that PMTPBA could serve as a promising probe for colorimetric sensing of PA and quantification within the above range. More sensitive determination of PA was performed by fluorescence method. Fig. 1C compared the emission spectrum of PMTPBA in the absence and presence of various concentrations of PA. It showed a highly fluorescent intensity of PMTPBA with random-coil conformation in aqueous media (λem =530 nm). Upon addition of increasing amounts of PA, the fluorescence at 530 nm decreased gradually in intensity, and reduced by 93% when the concentration of PA attained 5 mmol/L (Fig. 1C). A good linear relationship between (I0/I-1) and the concentration of PA (R2 = 0.997, from 5 nmol/L to 1500 nmol/L) was also obtained (Fig. 1D). These spectral results demonstrated that fluorometric detection of PA could also be achieved with a turn-off mode and the limit of detection (LOD) was as low as 5.0×10-8 mol/L.

|
Download:
|
| Fig. 1. Variation in the (A) absorption and (C) emission spectra of PMTPBA in 10 mmol/L HEPES buffer (pH 7.4) with increasing concentrations of PA as indicated. (B) The relationship between the ratio of A540/A410 and the concentration of PA. (D) Stern–Volmer plots of PMTPBA upon titration with PA; I0 and I are the fluorescence intensity at 530 nm of PMTPBA in the absence and presence of PA, respectively. λex =410 nm. (A, B) [PMTPBA] = 1×10-4 mol/L and (C, D) 5×10-5 mol/L respectively. | |
To check the detection limit of the visual sensing of PA in aqueous solution by the naked eye, the absorption and emission color of PMTPBA with gradually increasing concentrations of PA in HEPES buffer were compared in Fig. 2. It was found that when the concentration of PA is in excess of 10 μmol/L, the solution color change could be clearly discerned; whereas remarkable changes of the solution color could not be distinguished in the case of [PA] 5 μmol/L. Therefore, 10 μmol/L was defined as the visual LOD for colormetric detection of PA. Furthermore, this value for fluorescent assay could be extended to 2.5 μmol/L by the aid of a hand-held black light (excitation with 365 nm). This magnitude is among the lowest reported for the detection of PAwith naked eyes (Table S1 in Supporting information).

|
Download:
|
| Fig. 2. Photographs of PMTPBA under (A) laboratory lighting and (B) 365 nm UV light irradiation in the presence of various amounts of PA (μmol/L) as indicated. | |
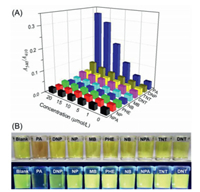
The specificity of PMTPBA toward PA was evaluated by examining the absorption spectra of PMTPBA in presence of various structural analogues under the same conditions including 2, 4-dinitrotoluene (DNT), nitrobenzene (NB), 4-nitrophenol (NP), 3-nitropropionic acid (NPA), methylbenzene (MB), phenol (PHE), 2, 4-dinitrophenol (DNP) and 2, 4, 6-trinitromethylbenzene (TNT). From Fig. S10 (Supporting information) we can see that the profiles of PMTPBA absorption spectra were barely affected by all of interferents except for PA, which caused a significant red-shifted of the absorption spectrum. The ratio of the absorbance at 540 nm to the absorbance at 410 nm, A540/A410 was calculated to further estimate the selectivity of PMTPBA towards PA (Fig. 3A). It was found that the ratio value in the presence of PA was approximately 4.4 times higher than that containing DNP, which is a major interferent in most sensing systems for PA. Distinct solution color changes can be also observed in Fig. 3B in which PA gave a dark orange and non-emissive solution, whereas other interferents remained yellow and high fluorescence. The similar results were obtained in the presence of common ions, such as K+, Ca2+, Mg2+, Na+, CO32–, NO3–, SO42–, and H2PO4–. The above results indicated that the PMTPBA is highly selective for the detection of PA. Compared with the previous reports on PA detection using conjugated polymers, PMTPBA exhibited competitive advantages in sensing performance including ease of preparation, excellent water solubility, high selectivity, low LOD, colorimetric and fluorescent dual detection (Table S2 in Supporting information). Especially, a large Ksv (7.15 105 L/mol) was obtained from the fluorescent responses of PMTPBA to PA, implying that PMTPBA featured a good binding affinity for PA in aqueous solution.
|
Download:
|
| Fig. 3. (A) The relative absorbance of PMTPBA in the presence of PA and other interferences under the identical conditions. (B) Photographs of PMTPBA responding to various nitro compounds in the absence (top) and presence (bottom) of UV light irradiation (365 nm). [PMTPBA] = 1×10-4 mol/L, [PA] = [all interferents] = 1.5×10-5 mol/L. | |
For understanding the molecular mechanism inside of these observations, we conducted a series of experiments and discussed the primarycauses of the colorimetric and fluorescent responses in the section that follows. The red-shifts in absorption spectrum and the color changes in solution indicated PMTPBA adopted more stretching conformations and ordered aggregates which originated from the formation of supramolecular complexes of PMTPBA and PA [33]. To confirm this point, the time-resolved decay of PMTPBA after the introduction of PA with various concentrations was inspected. Fig. S11 (Supporting information) showed that the lifetime of PMTPBA had hardly changed upon excitation with 440 nm, implying that the quenching of PMTPBA luminescence is due to a static mechanism, but not induced by a dynamic quenching procedure [34]. In this process, the complex between PMTPBA and PAwill be formed. Meanwhile, the Stern-Volmer plots at different temperatures were also depicted. It was found that fluorescence quenching efficiency decreased with the increasing temperature at the same concentration of PA (Fig. S12 in Supporting information). Additionally, the morphologies of PMTPBA before and after the addition of PA were examined by TEM. From Fig. S13 (Supporting information), we can catch sight of a large increase in particle size of PMTPBA in the presence of PA clearly. On the basis of the above results, the formation of nonemissive supramolecular assemblies induced by PA could be further confirmed.
With a view to acquiring in-depth understanding to the supramolecular complex between PMTPBA and PA and its forming mechanism in aqueous media, wecomparedthe spectral behaviors of PMTPBA responding to PA and its four analogues with similar chemical structures, TNT, NPA, DNP and NP (Fig. S14 in Supporting information). Firstly, given the fact that TNT (as the most similar compound of PA) did not induce any changes in absorption spectra of PMTPBA, one can conclude that electrostatic interactions originated from the negatively charged phenol group in PA and the positively charged ammonium group in PMTPBA would act as the key driving force for the supramolecular complexation of PMTPBA and PA. This point could be confirmed by ζ-potential measurements which were usually used for evaluating the status of surface charge of electrolytes and aggregates in solution. By this test, the original value of PMTPBA potential is 17.3 mV. With the addition of PA, it decreased to 0.03 mV. Since PA is highly acidic in nature, we also examined the fluorescence of PMTPBAunder acidic pH values for the sake of excluding the interference of pH changes. Fig. S15 (Supporting information) showed the emission intensityof PMTPBA at 530 nm was almost unchanged. Secondly, stoichiometry of the complex formation was also carried out on continuousabsorption plots from UV-vis spectrum. Fig. S16 (Supporting information) revealed that the absorbance at 540 nm (A540) increased by degrees with the rise of the molar fraction of PMTPBA (XPMTPBA). A maximum could be reached around XPMTPBA = 0.80, corresponding to the molar ratio of 4/1 (PMTPBA/PA). This value obviously deviated from the ratio of complementary electrostatic interaction between PMTPBA and PA, signifying that the cooperative effects of multi-noncovalent interactions would be in charge of this ratio and stable homogeneous dispersion of supramolecular complexes in aqueous media was preserved by the extra positive charges. This result is consistent with that of ζ-potential measurements. Furthermore, the negative results of DNP, NP (lack of nitro groups) and NPA (lack of aromatic rings) indicated that π-π stacking and charge transfer interactions were also the crucial factors in the fluorescence quenching and formation of supramolecular complexes. To further confirm the above inference, cyclic voltammetry measurements were conducted. HOMO and LUMO level related to PMTPBA were calculated to be -4.92 eV and -2.34 eV, respectively. Scheme S1 (Supporting information) compared the frontier orbital energies of PMTPBA and PA. It revealed that the LUMO energy of PMTPBA is higher than that of PA (-3.89 eV) [26], demonstrating electron transfer from PMTPBA to PA. 1H NMR spectra of PMTPBA, PA and their mixture were recorded in D2O solvent (Fig. S17 in Supporting information). It was shown that the protons in aromatic ring of PA and PMTPBA got hardly visible in the mixture in contrast to their alone in solution, supporting the role of strong π-π interactions in the complex. Stated thus, supramolecular complexes of PMTPBA and PA have been formed through the cooperative of electrostatic, charge transfer and aromatic stacking interactions, thereby leading to colorimetric and fluorometric dual responses.
To elucidate the feasibility of PMTPBA for sensing of PA in realistic samples, we investigated the sensing performance of PMTPBA toward surfactants [sodium dodecyl sulfate (SDS), hexadecyl trimethyl ammonium bromide (CTAB), tween 80] and common organic acids [oxalic acid (OA), formic acid (FA), citric acid (CA), malic acid (MA), acetic acid (AA)] in soil and water. From Fig. S18 (Supporting information) we can see that the above analytes barely affected the fluorescence of PMTPBA. Then, spiking tests were conducted in drinking water and soil samples. Absorption and emission spectra of PMTPBA in these samples were examined (Fig. S19 in Supporting information). The results showed that the spectral responses of PMTPBA are very similar with those observed in HEPES buffer. A good linearity between the ratio of A540/A410 and [PA], (R2 = 0.995 from 0.1 μmol/L to 9.0 μmol/L for drinking water, 0.997 from 0.1 μmol/L to 15 μmol/L for soil sample) was obtained. The detection limit obtained from fluorescence data can be lowered to 25 nmol/L in drinking water and 0.57 ppm in soil sample, respectively. It is demonstrated that PMTPBA could be applied to quantitative sensing of PA in environmental and food samples.
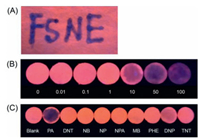
Prompted by the outstanding sensing properties of PMTPBA, were attempted to fabricate test strips with PMTPBA for portable detection of PA. For this purpose, filter papers were immersed in the PMTPBA solution for one minute, and then drying out to prepare the fluorescent test strips. Fig. 4A showed the fluorescent responses of the strip sensor caused by PA upon the excitation of a portable UV lamp. Strong red fluorescence could be observed on the blank paper strip upon irradiation at 365 nm, whereas it became dark when PA solutions were dripped onto the paper sensor. It could be clearly discernible by naked eyes due to the quenching of PMTPBA emission. To determine the visual LOD of PA using as-prepared paper strips, sample solutions with various amounts of PA were dripped onto these paper-based sensors. Fig. 4B exhibited that with the increasing concentrations of PA, the luminescence of paper strips became weaker. When [PA]≥10 μmol/L, changes of the strip fluorescence could be observed obviously. Therefore, 10 μmol/L was set to be the low visual LOD towards PA. Selectivity of test strips toward PA was also estimated in Fig. 4C. It was found that only PA significantly quenched the bright fluorescence of the strip, whereas other interferences barely induce the emission change. These results confirmed that PMTPBA can be embedded into an excellent strip-based probe in field testing with high sensitivity and selectivity. As we known, this is the first example for P3RO-4MeTs fabricated to be test strips in solid support.
|
Download:
|
| Fig. 4. Photographs of the test strips upon irradiation with a portable UV lamp of 365 nm visual detection of PA by handwriting (A), concentration-dependent luminescence after dropping PA solutions (μmol/L) (B), dropping different analytes solutions with the same concentration (50 μmol/L) and volume (10 μL) as indicated (C). | |
In conclusion, we have designed a novel water-soluble polythiophene derivative for the rapid detection of PA with colorimetric and fluorescent dual signal modes. It features fast response, excellent water solubility, low cost, high selectivity and sensitivity. The sensing mechanism could be ascribed to the supramolecular complexes formed between PMTPBA and PA through the cooperative non-covalent interactions, including π-π stacking, electrostatic and charge transfer interactions. The LOD could be reached 5.0×10-8 mol/L in solution. This probe could also be fabricated to fluorescence test strips for immediately monitoring of PA and the visual LOD of paper-based strip can be as low as 10 μmol/L. We believe this approach would not only provide a new method for colorimetric and fluorescent dual sensing of PA, but also bring some useful information for design and construction of solid sensing system based on CPs.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 31871877), Chinese Universities Scientific Fund (Nos. 2018QC151, 2019TC036), the "Talents Scheme" Program of Beijing (No. 31050003), and the Research Fund Program of Guangdong Provincial Key Laboratory of Radionuclides Pollution Control and Resources (No. 2017B030314182).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.003.
| [1] |
H.Q. Liang, Z.Y. Yao, W.Q. Ge, et al., RSC Adv. 6 (2016) 38328-38331. DOI:10.1039/C6RA04080B |
| [2] |
S. Hussain, A.H. Malik, M.A. Afroz, P.K. Iyer, Chem. Commun. 51 (2015) 7207-7210. DOI:10.1039/C5CC02194D |
| [3] |
K.Z. Gao, Y.Q. Guo, Q.Y. Niu, et al., Sens. Actuators B 262 (2018) 298-305. DOI:10.1016/j.snb.2018.02.008 |
| [4] |
S. Sanda, S. Parshamoni, S. Biswas, S. Konar, Chem. Commun. 51 (2015) 6576-6579. DOI:10.1039/C4CC10442K |
| [5] |
K. Bauri, B. Saha, J. Mahanti, P. De, J. Polym. Sci. A:Polym. Chem. 8 (2017) 7180-7187. |
| [6] |
A.S. Tanwar, P.K. Iyer, ACS Omega 2 (2017) 4424-4430. DOI:10.1021/acsomega.7b00765 |
| [7] |
H. Rapp-Wright, G. McEneff, B. Murphy, et al., J. Hazard. Mater. 329 (2017) 11-21. DOI:10.1016/j.jhazmat.2017.01.008 |
| [8] |
S.S.B. Moram, C. Byram, S.N. Shibu, B.M. Chilukamarri, V.R. Soma, ACS Omega 3 (2018) 8190-8201. DOI:10.1021/acsomega.8b01318 |
| [9] |
C. Crespy, P. Duvauchelle, V. Kaftandjian, F. Soulez, P. Ponard, Nucl. Instrum. Methods Phys. Res. A 623 (2010) 1050-1060. DOI:10.1016/j.nima.2010.08.023 |
| [10] |
A. Ramachandran, A.J.S. Nair, S.K. Yesodha, ACS Sustainable Chem. Eng. 7 (2019) 6732-6743. DOI:10.1021/acssuschemeng.8b05996 |
| [11] |
M. Calcerrada, M. González-Herráez, C. García-Ruiz, Trac-Trend. Anal. Chem. 75 (2016) 75-85. DOI:10.1016/j.trac.2015.08.005 |
| [12] |
U. Gaik, M. Sillanpaa, Z. Witkiewicz, J. Puton, Anal. Bioanal. Chem. 409 (2017) 3223-3231. DOI:10.1007/s00216-017-0265-2 |
| [13] |
A.S. Tanwar, L.R. Adil, M.A. Afroz, P.K. Iyer, ACS Sens. 3 (2018) 1451-1461. DOI:10.1021/acssensors.8b00093 |
| [14] |
H.B. Zhu, Y. Shen, Z.Z. Fu, et al., Chem. Commun. 103 (2019) 21-24. |
| [15] |
R. Goswami, N. Seal, S.R. Dash, A. Tyagi, S. Neogi, ACS Appl. Mater. Interfaces 11 (2019) 40134-40150. DOI:10.1021/acsami.9b15179 |
| [16] |
B.X. Zhang, H.J. Zhang, M. Zhong, Chin. Chem. Lett. 31 (2020) 133-135. DOI:10.1016/j.cclet.2019.05.061 |
| [17] |
X.F. Wu, H. Hang, H. Li, et al., Mater. Chem. Front. 1 (2017) 1875-1880. DOI:10.1039/C7QM00173H |
| [18] |
X.F. Wu, H. Tong, L.X. Wang, Prog. Chem. 31 (2019) 1509-1527. |
| [19] |
R. Sodkhomkhum, M. Masik, S. Watchasit, et al., Sens. Actuators B 245 (2017) 665-673. DOI:10.1016/j.snb.2017.01.120 |
| [20] |
J.Y. Du, J.P. Liu, Y.F. Ren, et al., Spectrochim. Acta A 211 (2019) 287-290. DOI:10.1016/j.saa.2018.12.014 |
| [21] |
H. Li, X.F. Wu, H. Hang, et al., Acta Polym. Sin. 2 (2018) 248-256. |
| [22] |
A.S. Tanwar, S. Hussain, A.H. Malik, M.A. Afroz, P.K. Iyer, ACS Sens. 1 (2016) 1070-1077. DOI:10.1021/acssensors.6b00441 |
| [23] |
A. Kalita, S. Hussain, A.H. Barman, et al., ACS Appl. Mater. Interfaces 8 (2016) 25326-25336. DOI:10.1021/acsami.6b08751 |
| [24] |
A.S. Tanwar, S. Patidar, S. Ahirwar, S. Dehingia, P.K. Iyer, Analyst 144 (2019) 669-676. DOI:10.1039/C8AN01970C |
| [25] |
T.M. Geng, S.N. Ye, Y. Wang, et al., Talanta 165 (2017) 282-288. DOI:10.1016/j.talanta.2016.12.046 |
| [26] |
A.H. Malik, S. Hussain, A. Kalita, P.K. Iyer, ACS Appl. Mater. Interfaces 7 (2015) 26968-26976. DOI:10.1021/acsami.5b08068 |
| [27] |
S.M. Tawfik, M. Sharipov, S. Kakhkhorov, M.R. Elmasry, Y. Lee, Adv. Sci. 6 (2019) 1801467. DOI:10.1002/advs.201801467 |
| [28] |
K. Lee, L.K. Povlich, J. Kim, Analyst 135 (2010) 2179-2189. DOI:10.1039/c0an00239a |
| [29] |
L.M. Guo, Z.Q. Zhang, Y.L. Tang, Chin. Chem. Lett. 29 (2018) 305-308. DOI:10.1016/j.cclet.2017.08.032 |
| [30] |
Z.Y. Yao, B.H. Huang, X.P. Hu, et al., Analyst 138 (2013) 1649-1652. DOI:10.1039/c3an00151b |
| [31] |
Z.Y. Yao, X.P. Hu, B.H. Huang, et al., ACS Appl. Mater. Interfaces 5 (2013) 5783-5787. DOI:10.1021/am401761n |
| [32] |
Y.L. Fan, X.W. Cheng, G.Y. Xue, J.B. Wu, Z.P. Huang, Spectrochim. Acta A 213 (2019) 210-217. DOI:10.1016/j.saa.2019.01.037 |
| [33] |
Z.Y. Yao, C. Li, G.Q. Shi, Langmuir 24 (2008) 12829-12835. DOI:10.1021/la802086d |
| [34] |
M.C. Rong, L.Q. Lin, X.H. Song, et al., Anal. Chem. 87 (2015) 1288-1296. DOI:10.1021/ac5039913 |
 2020, Vol. 31
2020, Vol. 31 

