b Key Laboratory of Analytical Chemistry of State Ethnic Affairs Commission, College of Chemistry and Materials Science, South-Central University for Nationalities, Wuhan 430074, China;
c Pharmaceutical College, Guangxi Medical University, Nanning 530021, China
Inherited disorders of amino acid metabolism in the neonatal period are diseases that are caused by impaired enzyme activity and genetic defects in the biosynthesis of transporters or cofactors [1-7]. Newborn screening programs (NBS) were thereby established by measuring content changes of amino acids for these amino acid metabolic disorders [1-7]. Direct infusion mass spectrometry (DIMS) methods have been served for screening amino acids (AAs) since 1990s [1-8]. However, some AAs may present low contents in the blood of newborns, such as citrulline (Cit) in newborns with short bowel syndrome or ornithine transcarbamylase deficiency (OTCD) [9, 10]. Unmatched concentration coverage for general DIMS methods led to unsuccessful diagnosis of these disorders. A more sensitive method for screening these AAs than DIMS methods is thereby demanded in this situation [10, 11]. Moreover, the traditional DIMS methods for screening AAs may sometimes get false-positive results due to complex matrices in dried blood spot (DBS) specimens, and they thereby required secondary test with blood resampling [3, 6, 7, 11]. Thus, improving the sensitivity and specificity of methods for screening of AAs is helpful for screening AAs with lower contents and supporting the secondary test without further blood sampling.
Derivatization assisted liquid chromatography tandem mass spectrometry (LC-MS) method has been used in DBS analysis with improved sensitivity and good selectivity in comparison with DIMS methods [1, 10, 12, 13]. Different derivatization reagents have been employed for screening inherited amino acid diseases or disorders [1, 10, 11, 14, 15]. For example, Trinh et al. reported the derivatization reagent N, N-dimethylformamide dimethylacetal (DMF-DMA) reacted with glutamine (Gln) for monitoring OTCD [10]. Tamashima et al. used 17-fluoronetaldehyde to derivatize 3 AAs for the simultaneous analysis of phenylketonuria (PKU) and maple syrup urine disease (MSUD) [11]. The detection sensitivity of AAs with poor ionization efficiency in ESI-MS can be enhanced to a certain extent after derivatization [10-12, 14, 15]. And AAs can be simultaneously measured for diagnosis of different inherited amino acid diseases or disorders with derivatization [3, 11, 12, 14, 15]. However, there are some limitations on the developmentof thesemethodsfor covering more diagnostically important AAs [14, 15]. The specificity of the derivatization reaction is insufficient, such as butanol, which reacts with both organic acids and oligosaccharides [10, 15-17]. The internal standards with stable isotope labelled amino acids (SILAAs) were very expensive [11, 18, 19]. Although analogues of amino acids were used to replace the expensive SIL-AAs in some studies [14, 20], it may decrease the reproducibility and reliability over methods with SIL-AAs [20]. It has been reported that stable isotope derivatization (SID) reagents can be used to overcome these problems [21-25]. The light ([1H]-incorporated) reagent used to derivatize samples and analyte derivatives of the heavy ([2H]- incorporated) reagent served as the stable isotope internal standards.
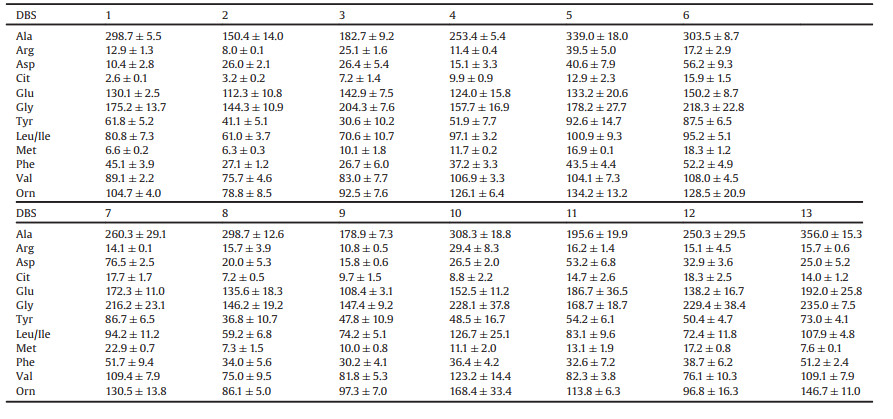
In this study, we establish a SID-LC-MS method with improved sensitivity and specificity for screening 12 diagnostically important AAs (Scheme 1). A pair of stable isotope reagent, p- (dimethylamino)phenyl isothiocyanate (DMAP-NCS) and 4-isothiocyanato-N, N-bis(methyl-[2H2])aniline ([2H4]DMAP-NCS) were employed. After evaluation of the assay, a simultaneously screening method for 12 amino acids in DBS specimens was established by using DMAP-NCS as the derivatization reagent to improve the sensitivity and specificity. And [2H4]DMAP-NCS derivatives of AAs ([2H4]DMAP-NCS-AAs) served as the internal standards in LC-MS detection for improving the detection accuracy. The established method was successfully applied in the analysis of AAs from DBS specimens.
|
Download:
|
| Scheme 1. Schematic for (a) derivatization reaction and (b) simultaneous analysis of amino acids (AAs) by DMAP-NCS and [2H4]DMAP-NCS derivatization assisted LC-MS method. | |
Isothiocyanates have been proven to be efficient for derivatizing amino group-containing compounds (-NH2) [14, 26, 27]. Firstly, we synthesized two new derivatization reagents, 2-(4-isothiocyanatophenyl)isoindoline-1, 3-dione (PIID-NCS) and methyl 4-isothiocyanatobenzoate (MePh-NCS), and compared with two commercial available derivatization reagents, DMAP-NCS and naphthyl isothiocyanate (ANIT). The reaction reactivity was tested between the investigated reagents and amino acids. The results showed that PIID-NCS and MePh-NCS only reacted with amino acids under extremely harsh condition (DMF as solvent, 110 ℃ reflux overnight), indicating they were not suitable for derivatization. ANIT reacted with all the amino groups in the diamino acid, ornithine (Orn). Both [ANIT + Orn+H]+ ion and [2ANIT + Orn+H]+ ion could be observed in positive mode of ESI-MS, while only [DMAP-NCS + Orn+H]+ ion for DMAP-NCS derivatization (Fig. S1 in Supporting information). The detection sensitivity of ANIT derivatives of AAs were also lower than DMAP-NCS derivatives of AAs. Therefore, DMAP-NCS was finally selected as the derivatization reagent for AAs.
Previous publications presented various reaction conditions for isothiocyanate derivatization, such as pyridine/ethanol condition, acetonitrile/water/triethylamine condition [14, 26, 27]. Multiple derivatives can be observed for DMAP-NCS derivatization under pyridine/ethanol condition (Fig. S2 in Supporting information). The AA derivatives of DMAP-NCS degraded into their dehydration products under acetonitrile/water/triethylamine condition (Figs. S3b and c in Supporting information). Our results showed that only one derivative was observed under Na2CO3/NaHCO3 buffer as the alkaline catalyst (Figs. S3d and e in Supporting information). The reaction selectivity under Na2CO3/NaHCO3 buffer was then examined using methanethiol (-SH), 1-octanol (-OH), and 1-naphthylamine (-NH2) as the reaction substrates. No reaction products of DMAP-NCS with -SH and -OH under Na2CO3/ NaHCO3 buffer were observed (Fig. S4 in Supporting information). These reaction substrates were performed DMAP-NCS derivatization again and analyzed by ESI-MS. In the recorded full scan mass spectrum, the intense peak of derivatized 1-naphthylamine ion was observed (Fig. S5 in Supporting information), while the characteristic ions for methanethiol and 1-octanol derivatives with DMAP-NCS were not detected. These collective results indicate DMAP-NCS reacted selectively towards -NH2.
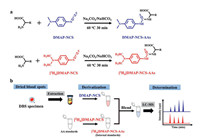
To achieve high derivatization efficiency, the derivatization reaction conditions including the water content, pH value of Na2CO3/NaHCO3 buffer, reaction temperature, and reaction time were optimized. When the proportion of water continued to increase, turbidity was produced due to weak polarity of DMAPNCS. The proportion of water was thereby controlled to be within 60% (v/v) (Fig. 1a). The pH value of the buffer was then tested in the range of 5.0–11.0 (Fig. 1b). The reaction temperature was evaluated at the range of 30-70 ℃ (Fig. 1c), and the reaction time ranged from 10 min to 55 min (Fig. 1d). After careful examination, the optimal reaction conditions were selected as follows: 30 min at 60 ℃ under Na2CO3/NaHCO3 buffer at pH 9.0 (Fig. 1). The amount of DMAP-NCS was then investigated in QC samples. The concentration of DMAP-NCS for derivatizing DBS samples was found to be at 5 mg/mL (Fig. 1e). The stability of the AA derivatives of DMAP-NCS was then investigated by monitoring the peak areas of them every one hour under MRM mode. The results showed that the relative standard deviations (RSDs, %) of peak area for the derivatives are less than 4.6% within 18 h (Fig. 1f).

|
Download:
|
| Fig. 1. Optimization of derivatization reaction conditions of DMAP-NCS towards amino acids (AAs), including (a) pH, (b) water contents, (c) reaction temperature, (d) reaction time, (e) concentration of DMAP-NCS in DBS specimen and (f) short-term stability of DMAP-NCS-AAs. | |
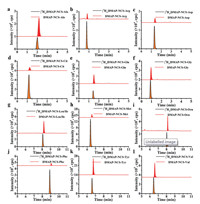
After DMAP-NCS derivatization, the chromatographic separation of AAs was significantly improved on C18 column (Fig. 2). The [2H4]DMAP-NCS-AAs were prepared from [2H4]DMAP-NCS at the optimal reaction conditions and served as the internal standards. We then compared the isotopic effects of DMAP-NCS-AAs with [2H4] DMAP-NCS-AAs. The isotopic effects were found to be less than 0.2 min with same elution condition on the C18 column (Fig. 2).
|
Download:
|
| Fig. 2. MRM chromatograms of DMAP-NCS derivatives of (a) Ala, (b) Arg, (c) Asp, (d) Cit, (e) Glu, (f) Gly, (g) Leu/Ile, (h) Met, (i) Orn, (j) Phe, (k) Tyr and (l) Val from extracts of a DBS specimen. | |
For method development, selectivity and carryover were firstly evaluated. There were essentially no interferences produced in the extracts of cards without spotting blood during LC-MS analysis (Figs. S6a-c in Supporting information). The analysis of quality control (QC) samples can be made without significant interferences for the derivatives of [2H4]DMAP-NCS, indicating good selectivity for the derivatization method (Figs. S6d-i in Supporting information). No carry over effect was observed after the analysis of upper limit of quantification (ULOQ) QC samples followed by extracts from blank cards. The AAs in the blood may interact with the sampling paper and lead to the concentration different between the center and edge of the spots. It was studied the distribution effect and the results showed that the RSDs for the concentration of AAs in the 7 punching positions were less than 15%, suggesting that chromatographic effect or possible inhomogeneity is negligible (Fig. S7 in Supporting information) [28]. With fixed punching size and red blood cell counts, the blood volume may also result in deviations for the concentrations. Reduced deviations for the peak area ratios of QC samples was observed when the sample volume was greater than 50.0 μL (Fig. S8 in Supporting information). The matrix effect of the derivatization method was evaluated to be in the range of 91.0%–103.2% (Table S1 in Supporting information).
Calibration curves were conducted from the peak area ratios of DMAP-NCS-AAs at different concentrations to [2H4]DMAP-NCSAAs. The results showed that the determination coefficient (r2) was greater than 0.99, the LODs for the investigated AAs ranged from 0.1 nmol/L to 13.0 nmol/L, and the LOQs were in the range of 0.3–43.3 nmol/L (Table S2 in Supporting information). In order to verify the stability and accuracy of the method, 12 AAs with different concentrations were added into the pooled whole blood samples of 6 newborns to prepare the QC samples. The intra-day and inter-day precision of the method was investigated with these QC samples, and their RSDs were less than 16.3%. The recoveries were found to be in the range of 72.4%–128.1% (Table S3 in Supporting information).
Dilution of the sample extracts was the conventional approach for samples with the concentrations of AAs exceeding the ULOQs. The dilution factors of all 12 AAs were less than 15.6%, and the RSDs between contents of AAs for repeated samples were less than 20% (Table S4 and Fig. S9a in Supporting information). Therefore, for ULOQ samples, quantitative analysis can be performed by diluting the extracts of these samples. The accuracy and reliability of the results for the method was also evaluated by incurred sample reanalysis. The RSDs between the repeated sample results and the original sample results after 6 days were less than 15% (Table S4 and Fig. S9b in Supporting information), which were inside the ±20% window. It meets the requirements of DBS sample reanalysis [28].
We compared the proposed method with other reported LC-MS methods for DBS specimens, in terms of the types of AAs, LODs, and the types of internal standards (Table S5 in Supporting information). The established method in this study can simultaneously analyze 12 diagnostically important AAs in a single run and have better detection sensitivity. The enhanced sensitivity of the method allowed the dilution of the extracts from DBS specimens, which was beneficial to minimize the matrix effects [29]. Previous studies usually purchased expensive SIL-AAs as the internal standards. In this work, we only need to synthesize the [2H4] DMAP-NCS reagent and label AA standards with [2H4]DMAP-NCS to prepare stable isotope internal standards [2H4]DMAP-NCS-AAs.
The proposed method was finally applied to detect AAs in DBS specimens from healthy newborns (n = 13). Fig. 2 shows the representative MRM chromatograms of 12 AAs detected in a DBS specimen. There was no observation of significant interferences. Table 1 summarizes the contents of the investigated AAs in the DBS specimens. Screening profiles of the AAs were also supplied in Supporting information, including their associated metabolic disorders and the normal cutoff values (n.v.) (Table S6 in Supporting information). Take phenylalanine (Phe) and tryrosine (Tyr) as examples, the abnormal contents of these two AAs in newborns were associated with classic PKU. The molar ratio of Phe toTyr (Phe: Tyr) was also assigned as the indicator for classic PKU. From our results, the contents of Phe and Tyr were in the range of 27.1– 52.2 μmol/L and 30.6–92.6 μmol/L, respectively. The molar ratio of Phe to Tyr ranged from 0.5 to 0.9. These values were within the cut off values of contents and molar ratios for newborn population, which suggested that none of the 13 newborns had classic PKU. These results indicated that the proposed method could be used for diagnosis of classic PKU in DBS specimens from newborns.
|
|
Table 1 Amount of amino acids (μmol/L) in dried blood spots of newborn infants (n = 13). |
In conclusion, the established SID-LC-MS method allowed the simultaneous screening of 12 amino acid from DBS specimens. After derivatizing with DMAP-NCS, the investigated 12 AAs can be separated within 12 min with acceptable resolution. The method improved the sensitivity of these AAs down to pmol/L level during LC-MS analysis. The enhanced sensitivity led to reduce the sample volume of blood, which may be beneficial for reducing the pain of infants during blood sampling. A stable isotope reagent [2H4] DMAP-NCS was synthesized and its derivatives of AAs were utilized as the internal standards. Synthesis procedure of [2H4] DMAP-NCS was simple, and its cost-price is much lower than that of SIL-AAs. The introduction of stable isotope derivatization reagent for AAs can allow screening of AAs without relying on the commercial NBS kits. We hope that our assay may be applied for the AAs screening, especially for those lack of SIL-AAs or for the specific examination in case of suspected amino acid metabolic disorders.
Declaration of competing interestWe declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
AcknowledgmentsThe work is supported by the National Key R&D Program of China (No. 2018YFA0900400), the National Natural Science Foundation of China (Nos. 21635006, 31670373, 21721005, 21904099), and the Postdoctoral Science Foundation of China (No. 2018M642893).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.003.
| [1] |
D.H. Chace, D.S. Millington, N. Terada, et al., Clin. Chem. 39 (1993) 66-71. DOI:10.1093/clinchem/39.1.66 |
| [2] |
M. Champion, Arch. Dis. Child. Educ. Pract. Ed. 95 (2010) 40-46. DOI:10.1136/adc.2008.151183 |
| [3] |
G. la Marca, J. Pharm, Biomed. Anal. 101 (2014) 174-182. DOI:10.1016/j.jpba.2014.03.047 |
| [4] |
E. Scolamiero, C. Cozzolino, L. Albano, et al., Mol. Biosyst. 11 (2015) 1525-1535. DOI:10.1039/c4mb00729h |
| [5] |
M. Wagner, D. Tonoli, E. Varesio, G. Hopfgartner, Mass Spectrom. Rev. 35 (2016) 361-438. DOI:10.1002/mas.21441 |
| [6] |
D. Ombrone, E. Giocaliere, G. Forni, S. Malvagia, G. la Marca, Mass Spectrom. Rev. 35 (2016) 71-84. |
| [7] |
C. Bruno, D. Dufour-Rainfray, F. Patin, et al., Clin. Biochem. 49 (2016) 1047-1050. DOI:10.1016/j.clinbiochem.2016.07.008 |
| [8] |
L. Dai, N. Guo, Y. Liu, et al., Chin. Chem. Lett. 30 (2019) 103-106. DOI:10.1016/j.cclet.2017.12.023 |
| [9] |
N. Janzen, M. Terhardt, S. Sander, et al., Clin. Chim. Acta 430 (2014) 28-32. DOI:10.1016/j.cca.2013.12.020 |
| [10] |
M.U. Trinh, J. Blake, J.R. Harrison, et al., Clin. Chim. 49 (2003) 681-684. DOI:10.1373/49.4.681 |
| [11] |
E. Tamashima, T. Hayama, H. Yoshida, et al., J. Pharm. Biomed. Anal. 115 (2015) 201-207. |
| [12] |
M. Star-Weinstock, B.L. Williamson, S. Dey, S. Pillai, S. Purkayastha, Anal. Chem. 84 (2012) 9310-9317. DOI:10.1021/ac302036r |
| [13] |
N. Hamada, Y. Hashi, S. Yamaki, et al., Chin. Chem. Lett. 30 (2019) 99-102. |
| [14] |
Y. Dale, V. Mackey, R. Mushi, et al., J. Chromatogr. B 788 (2003) 1-8. DOI:10.1016/S1570-0232(02)01005-X |
| [15] |
V.R. de Jesús, D.H. Chace, T.H. Lim, J.V. Mei, W.H. Hannon, Clin. Chim. Acta 411 (2010) 684-689. DOI:10.1016/j.cca.2010.01.034 |
| [16] |
P.J. Trim, J.J. Hopwood, M.F. Snel, Anal. Chem. 87 (2015) 9243-9250. |
| [17] |
X. Zhou, S. Yang, G. Yang, Z. Tan, F. Guan, Chin. Chem. Lett. 30 (2019) 676-680. DOI:10.1016/j.cclet.2018.12.016 |
| [18] |
R. Dahl-Lassen, J. van Hecke, H. Jørgensen, et al., Plant Methods 14 (2018) 8. DOI:10.1186/s13007-018-0277-8 |
| [19] |
C. Wadham, A.A. Mangoni, Expert Opin. Drug Metab. Toxicol. 5 (2009) 303-319. DOI:10.1517/17425250902785172 |
| [20] |
C. Wang, W. Zhang, F. Song, Z. Liu, S. Liu, Amino Acids 42 (2012) 1889-1895. DOI:10.1007/s00726-011-0910-6 |
| [21] |
M. El-Maghrabey, N. Kishikawa, N. Kuroda, Anal. Chem. 90 (2018) 13867-13875. DOI:10.1021/acs.analchem.0c00044 |
| [22] |
N. Guo, P. Liu, J. Ding, et al., Anal. Chim. Acta 905 (2016) 106-114. DOI:10.1016/j.aca.2015.12.010 |
| [23] |
Y.H. Hao, Z. Zhang, L. Wang, et al., Talanta 144 (2015) 341-348. DOI:10.1016/j.talanta.2015.06.056 |
| [24] |
Q.F. Zhu, Y.H. Hao, M.Z. Liu, et al., J. Chromatogr. A 1410 (2015) 154-163. DOI:10.1016/j.chroma.2015.07.100 |
| [25] |
M.H. El-Maghrabey, N. Kishikawa, N. Kuroda, Biomed. Chromatogr. 34 (2020) e4756. |
| [26] |
T. Santa, Biomed. Chromatogr. 24 (2010) 915-918. DOI:10.1002/bmc.1352 |
| [27] |
G. Zheng, W. Jin, P. Fan, et al., Int. J. Mass Spetrom. 392 (2015) 1-6. DOI:10.1016/j.ijms.2015.08.004 |
| [28] |
W. Li, Considerations in development and validation of LC-MS/MS method for quantitative analysis of small molecules in dried blood spot samples, in: W. Li, M.S. Lee (Eds.), Dried Blood Spots: Applications and Techniques, John Wiley & Sons, Hoboken, 2014, pp. 168-178. http://www.researchgate.net/publication/277690410_Considerations_in_Development_and_Validation_of_LC-MSMS_Method_for_Quantitative_Analysis_of_Small_Molecules_in_Dried_Blood_Spot_Samples
|
| [29] |
H. Bagheri, O. Zandi, A. Aghakhani, Anal. Chim. Acta 716 (2012) 61-65. DOI:10.1016/j.aca.2011.10.033 |
 2020, Vol. 31
2020, Vol. 31