b Chemistry and Chemical Engineering Guangdong Laboratory, Guangdong Technion-Israel Institute of Technology (GTIIT), Shantou 515031, China;
c State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, China;
d State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China;
e Department of Chemistry and Laboratory of Advanced Materials, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200433, China
The current challenges facing society have propelled the demand for synthetic methods that are resource-efficient, safe and environmentally friendly. Recently, micro-reaction technology has gained significant popularity because of the dramatic differences in material performance at the microscopic scale, such as precisely tuning diffusion and mixing, fast mass/heat transport and inherent safety. Methods involving high synthesis efficiency through miniaturization have resulted in remarkable achievements not only in academic research areas but also with industrial applications [1, 2]. On April 1 2019, the International Union of Pure and Applied Chemistry (IUPAC) released the top ten emerging technologies in chemistry (this is the first time for IUPAC to identify ten chemical innovations that will change our world). Flow chemistry, one of those technologies with potential to make our planet more sustainable, was on the list.
Beyond those existing uses in the fields of organic synthesis, analytic chemistry and the pharmaceutical industry, it is also conceivable that the combination of this method with other techniques such as photochemistry might revolutionize synthetic methods. As a "clean and traceless reagent", light of different wavelengths could be used as the driving force for chemical reactions.
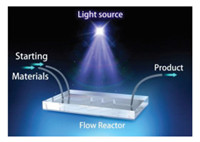
Continuous-flow photochemistry, namely flow reaction under light irradiation, facilitates reduced reaction time, improves masstransfer characteristics, renders scalability more straightforward and allows for cost-effective harvesting of the product (Fig. 1) [3]. The high surface-to-volume ratio of the flow-type tube could be used to maximize illumination and help transfer heat effectively while addressing the issue of thermal side products. Integrated with the advantages of flow chemistry and photochemistry, this technique is emerging as a green and sustainable technology [4-8].
|
Download:
|
| Fig. 1. A representative scheme of continuous-flow photochemistry. | |
This review was based on the sustainable technology point of view and most representative examples were published after 2016. The application of continuous-flow photochemistry in organic synthesis, material science and water treatment till 2016 was summarized [3]. There are nine good reasons for researchers to utilize this technique. Apart from intrinsic characteristics such as: (1) fast mixing, (2) fast heat exchange, 3) multiphase chemistry, (4) reliable scale-up and (5) increased safety, other remarkable factors such as (6) improved irradiation of the reaction mixture, (7) improved reaction selectivity and reproducibility, (8) multistep reaction sequences and (9) immobilized catalysts are of vital importance to a range of sustainable chemical reactions. These important characteristics make this technique very promising in a wide variety of applications. Loubière and colleagues summarized the valuable characteristics of this method, and readers interested in the chemical engineering principle should refer to their article [9]. Recent research has further demonstrated the synergistic effect of flow chemistry and photochemistry, which are provided by this protocol [10, 11]. To illustrate these benefits, some examples from the literature will be elaborated in the following paragraphs.
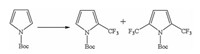
Taking into account the improvement of metabolic stability and pharmacokinetic properties, introducing the trifluoromethyl group into drug molecules has become an important field of research in the pharmaceutical industry. To scale up, given the diverse synthetic routes such as radical, nucleophilic and electrophilic reactions [12], obstacles relating to costly and unobtainable raw materials in required quantities could be circumvented by using continuous-flow photochemistry. Stephenson and colleagues have used a scalable and operationally simple radical trifluoromethylation reaction by utilizing inexpensive trifluoroacetic anhydride (TFAA) as a CF3 source (Fig. 2) [13].
|
Download:
|
| Fig. 2. The reaction scheme of trifluoromethylation by using continuous-flow photochemistry. | |
A mixed acetonitrile solution (20 g) of tris(bipyridine)ruthenium(II) chloride (photocatalyst), pyridine N-oxide (sacrificial redox reagent), the substrate N-Boc-pyrrole (Boc = tert-butyloxycarbonyl) and TFAA was flowed in the reactor (10 mL volume) under blue light-emitting diodes (LED) light with a reaction time of 10 min to afford 71% yield (mixture of the mono-functionalized and doubly functionalized product with the ratio of 3:2), demonstrating the advantage of using highly efficient light irradiation of the reaction solution. In comparison, a similar reaction scale (18 g) under the conventional batch type gives 57% yield with long reaction time of ~15 h. Additionally, higher intensity light sources could increase both reaction rates and yield. More recently, Harper and colleagues developed a laser driven continuous stirred tank reactor (100 mL) to drive photochemical cross-coupling reactions at high rates, fabricating products with a scale of a kilogram per day [14]. Unravelling the intrinsic relationship between light source and reaction rate could help to design versatile flow reactors with the possibility of scaling-up. The reaction of photocatalytic oxidation of methionine toward methionine sulfoxide has been explored in industrial scale, namely, a Corning advanced-flow G1 photoreactor could produce the final product with the productivity of 5.1 kg per day whereas 6 tons products could be obtained in a day by using a Corning advanced-flow G3 photoreactor [15]. Another recent example regarding visible light induced nickel Negishi reaction has been reported by using a Corning G1 photoreactor, with the maximum level of products could reach to 270 g per day with the aim to support preclinical medicinal experiments [16]. Those examples have demonstrated that continuous-flow photochemistry was a practical and efficient technology in industrial field.
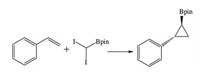
In a similar manner, using xanthone as an organic photosensitizer and N, N-diisopropylethylamine (Hünig's base), the photoredox borocyclopropanation reaction of styrenes derivatives has also been developed under flow type [17]. Starting with styrene and diiodomethylpinacol (I2CHBpin) via a Simmons-Smith reaction, using a custom-made photoreactor consisting of UV-A lamps (350 nm) and flow reactors, the borocyclopropane product could be harvested at 78% yield with the residence time of 1 h (Fig. 3). On the contrary, no reaction was observed after 1 h using a batch type reactor, and the product obtained was at 58% yield after 18 h, illustrating the superior character of flow-type reactions.
|
Download:
|
| Fig. 3. The reaction scheme of borocyclopropanation under UV light under flow condition. | |
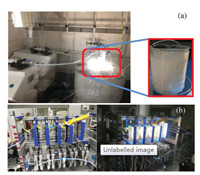
Apparently, the flow device is highly adapted with specific purpose in the application, those representative device photos have shown its characters from laboratory scale to industrial scale (Fig. 4).
|
Download:
|
| Fig. 4. (a) The custom-built apparatus in the Xie's laboratory: the tube is wrapped around the beaker; (b) left: A Corning G1 reactor with the LED lights, right: the LED lights are illuminating. | |
Using continuous-flow photochemistry to address environmental priorities such as indoor air pollutants and water contamination has been widely performed. For each scenario, this strategy is based on the photocatalytic process under flow type.
Air pollution harms humans and is a challenge to the goal of sustainable development. Particularly, indoor air pollution is one of the top environment risks, because, in general, people spend the majority of their time indoors and health effects such as the irritation of the nose and throat, headaches, dizziness, fatigue (immediate effects) and respiratory diseases, heart disease and cancer (long-term effects) may develop. For the most part, volatile organic compounds (VOCs) such as aliphatic and cyclic hydrocarbons, aromatic hydrocarbons, alkene, benzene, toluene, aldehydes and different inorganic compounds such as NOx, SOx and NH3 are the sources of indoor air pollution. Relying on continuousflow photochemistry, a flow system consisting of a solid semiconductor catalyst that is activated by photoradiation for air purification is recognized as a cost-efficient approach [18-20]. In this context, photocatalytic oxidation (PCO) is an innovative and promising approach given that most PCO reactors use anatase TiO2 as the catalyst that is activated by UV light.
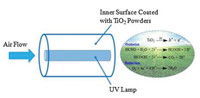
By virtue of the annular reactor that is coated with TiO2 powders, Zhang and colleagues described their efforts to remove VOCs under flow in an effective way (Fig. 5) [21]. Formaldehyde was selected as the target pollutant as this compound is a typical indoor VOC that is commonly found in modern building materials and household products. Activated by ultraviolet (UV) light, the TiO2 catalyst produces h+ and e-, which are powerful oxidizing and reducing agents, respectively. Consequently, organic compounds found in air such as formaldehyde, can be converted into benign and odorless complexes, i.e., H2O and CO2. The modification of TiO2, integrated with different types of flow reactors, could help to maximize the potential of continuous-flow photochemistry for indoor air purification [22-24].
|
Download:
|
| Fig. 5. (left) Schematic of the annular photocatalytic reactor; (right) Processes in the photodegradation of formaldehyde. Reproduced with permission [21]. Copyright 2008, Elsevier. | |
Water research is critical for a sustainable future, and identifying better solutions to current societal challenges including water pollution, is crucial. Because of the large surface-to-volume ratio of flow reactors, the efficiency of the mass transfer and photon transfer of photocatalytic water purification could be improved dramatically [25]. Notably, advanced oxidation processes (AOPs) in continuous-flow photochemistry have attracted research interest because they could serve as an efficient means for water decontamination [26]. By far, two strategies have been used the most when integrating with a flow reactor, namely, the catalysis by TiO2 and the iron catalyzed photo-Fenton reaction. Generally, the former is a heterogeneous photocatalysis and the latter is a homogeneous photocatalysis.
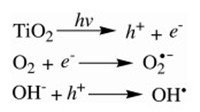
For conventional water treatment using AOPs, hydroxyl radicals (OH·) play an important role in degrading various types of contaminants due to their strong oxidative activity in aqueous solutions. The photoexcitation of TiO2 produces an electron-hole pair and consequently, superoxide and hydroxyl radicals are generated (Scheme 1).
|
Download:
|
| Scheme 1. Production of superoxide and hydroxyl radicals. | |
Using a custom-built photocatalytic reactor containing two TiO2-coated glasses as the top cover, Zhang and colleagues observed that the process of 30% degradation of the model pollutant, methylene blue, only took 5 min, with a reaction rate constant two orders higher than the bulk reactor [27]. As illustrated by these researchers, this sophisticated system has a potential to help tackle the problem of water pollutants given its high efficiency of mass/photon transfer.
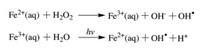
Compared to the TiO2 catalyst using UV light, the photo-Fenton reaction could be activated by visible light because of the absorbance of iron-hydroxy and iron-organic complexes in aqueous solution. Likewise, the super oxidative activity of hydroxyl radicals is the key for water decontamination (Scheme 2). Although the research on flow systems taking advantage of photo-Fenton reaction is scarce [28], chemists could find niche applications in purifying wastewater in the near future.
|
Download:
|
| Scheme 2. The process of photo-Fenton reaction. | |
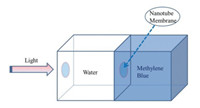
The Schmuki group has led a research initiative—flow-through photocatalysis taking advantage of nanotubes as a photoactive membrane—that aims to decontaminate toxics from water more effectively (Fig. 6) [29, 30]. Notably, Schmuki and colleagues have exploited the unique functional properties of TiO2 and fabricated vertically oriented, both-side-open nanotubes to achieve a freestanding membrane. The model pollutant (methylene blue) has been tested for its photocatalytic decomposition under UV light, demonstrating the effectiveness of TiO2 membrane for water purification [29]. More recently, the same group has fabricated both-side-open Ta3N5 nanotube membranes to degrade methylene blue under visible light or solar illumination [30]. It is anticipated that this approach using a range of sophisticated nanoscale materials to assemble micro-reactors could optimize the photocatalyst required for sustainable water remediation.
|
Download:
|
| Fig. 6. The scheme of flow-through photocatalytic experiments using nanotube membrane. Reproduced with permission [29]. Copyright 2007, ACS publications. | |
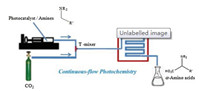
Global energy demands and climate change are the two great challenges facing the 21st century. As a climate-changing small molecule, CO2 has spawned broad-based research due to its low reactivity and pronounced stability, in addition to the possibility of its chemical transformation to commodity and high-added-value chemicals in an atom-efficient manner. Despite numerous fundamental problems that must be addressed in order to convert CO2 into valuable C1-Cn products and intermediates, the use of continuous-flow photochemistry in this competitive arena is relatively rare. In a recent publication, using photoredox activation in flow condition and through a single-electron reduction route, CO2 was found to couple with appropriately chosen amines to produce α-amino acids in a feasible way (Fig. 7) [31]. The incorporation of a commercially available organic photoredox catalyst, para-terphenyl, and the amine substrate into a flow system to mix with CO2 gas has evolved from intuitive efforts by Jamison, which foster the formation of carbon–carbon bonds in a flow reactor. The photoreduction of CO2 to α-amino acids involves two major steps: the single-electron reduction of CO2 and radical– radical coupling to produce the final products. The knowledge gained from this approach could enable the construction of different structures through C-H functionalization.
|
Download:
|
| Fig. 7. The setup of continuous-flow photochemistry for the photoredox-catalysed synthesis of α-amino acids. Reproduced with permission [31]. Copyright 2017, Springer Nature. | |
Taking advantage of 1, 2, 2, 6, 6-pentamethylpiperidine as a photocatalyst, under an atmospheric pressure of CO2, the direct β-selective hydrocarboxylation of styrenes was carried out under continuous-flow condition by the same group [32]. It should be noted that this strategy can be used to optimize parameters that do not readily vary in traditional batch-type conditions and, apparently, the short-path length of light and superior mixing of this technology could improve the efficiency of photochemical transformation.
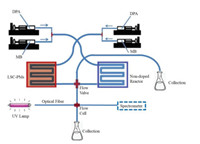
4.2. Solar energy utilizationDwindling supplies of fossil fuels have led to the quest for new, clean and sustainable energy sources. Converting solar light into chemical energy is one attractive avenue. An overarching theme has been to develop a device by which chemicals can react with sunlight in an efficient and inexpensive way. Similar to a leaf on a tree, a user-defined photomicroreactor was reported by Noël and colleagues (Fig. 8) [33]. Intriguingly, a luminescent solar concentrator (fluorescent dye Lumogen F red 305, LSC) associated with polydimethylsiloxane was used to build optimizing devices (LSCPhotoMicroreactors, LSC-PMs) consisting of microchannels capable of capturing direct and diffuse sunlight and then converting it into light with a narrower wavelength. When methylene blue (MB) was employed as the photocatalyst, based upon a pertinent benchmark reaction, i.e., the [4 + 2] cycloaddition of singlet oxygen to 9, 10-diphenylanthracene (DPA) in flow reaction, a converting efficiency (solar light to chemical energy) was reached as high as 10%. It is anticipated that solar-driven photocatalytic transformations under flow would be observed consistently from a number of targeted studies and these transformations provide a sufficient basis to incorporate continuous-flow photochemistry into a variety of sustainable technologies.
|
Download:
|
| Fig. 8. The setup employed for the solar experiment with the aim to compare the conversion in both the non-doped reactor and the LSC-PMs. Reproduced with permission [33]. Copyright 2017, Wiley-VCH. | |
The advancement of efficient flow reactors could develop into applications for biomedical and microfluidic devices since at the micro-scale, propelling liquids in a controllable manner is an important issue. More specifically, using the photochemical protocol to control the movement of liquids extends the scope of continuous flow photochemistry. Inspired by the structural character of arteries, Yu and colleagues developed a photodeformable tubular device that is triggered by standard LED light [34]. The composition is a linear liquid-crystal polymer, and the shape can be adjusted by light while maintaining its robustness. The photodeformation produces capillary forces such that a diversity of liquids moves forward when exposed to light and can even travel uphill. Previous reports have suggested that light-induced capillary forces can drive the movement of liquids. However, the slow speed of movement, the less suitable types of liquids and potential contamination by added photosensitive compounds create hurdles for their application. However, Yu and her colleagues demonstrated the speed at which the movement of hexane reaches 5.9 mm/s and a silicon oil slug could move a long distance (> 50 mm); they also employed a diversity of liquids including nonpolar and polar liquids, liquids for biomedical engineering, and complex fluids such as liquid–solid fluid mixtures and even petrol. Furthermore, they demonstrated feasibility without any contamination found. The uniqueness of this kind of device will surely be examined further by researchers studying micro-pumps, and this will underpin the development of new technologies, such as micro-reactors, laboratory-on-a-chip devices and micro-optomechanical systems.
Targeted drug delivery, providing effective pain relief by delivering medication directly, is a technology to satisfy patients with less or no need for oral medication. Intriguingly, Schmuki and colleagues have used Fe3O4-filled TiO2 nanotubes as a movable photocatalyst, integrating a violet-blue fluorescent marker as a model drug by cross-linking, to create an effective temporally and spatially controlled drug release system [35]. Irradiated by UV light, magnetic TiO2 nanotubes could release the model drug with site selectivity. Furthermore, taking advantage of its photocatalytic activity, the potential of this specific system to kill cancer cells (HeLa tumor cells) has been exploited.
5. Summary and outlookThe present work illustrates the benefits inherent in continuous-flow photochemistry combined with the advantages of flow chemistry and photochemistry. A number of recent developments working to tackle environmental issues such as indoor air pollution and wastewater, rising CO2 emissions, solar energy utilization and biomedical equipment could foster striking progress in sustainability.
As illustrated, the desire to improve quality of life by addressing air/water pollution has provoked intense research on the development of methods capable of tackling emerging contaminants. Results have demonstrated that continuous-flow photochemistry could help to achieve a healthier indoor environment and cleaner water, and it will continue to evolve and serve as a powerful strategy for environmental issues. Second, owing to its abundance and nontoxicity, CO2 could serve as an ideal carbon source to produce α-amino acids, which would be a sustainable approach to produce various organic compounds. Given the combination of utilizing gases and photochemistry in flow, photoreduction of CO2 is particularly appealing because this process proceeds under a flow-type reactor and can remove the scale limitations encountered with batch-type reactors. It is costeffective and has a minimal environmental impact, yet it can produce valuable chemicals. Third, to achieve environmental sustainability, the conversion of solar light into chemical energy in an effective and economically viable manner holds great potential. Through continuous-flow photochemistry, the converting efficiency (from solar light to chemical energy) has improved to a stage permitting us to fully exploit direct and diffuse sunlight when integrated with luminescent solar concentrators. Solar-driven continuous manufacturing of commodities could inspire new perspectives on using solar light as an affordable, reliable and secure source of energy. Last, in developed and developing countries alike, the need for smart solutions to manipulate biomedical devices is ever-increasing. The microtube can spontaneously change shape upon the LED light irradiation in a controlled manner and consequently, drive the liquid to move forward. Because light can provide contactless spatial and temporal control, this strategy of flow under light could facilitate a generation of biomedical devices with unprecedented functionalities. A few groups have made important inroads into continuous-flow photochemistry to target sustainability issues with new solutions.
The growing understanding of continuous-flow photochemistry has allowed more consideration of the energy efficiency of this technique. UV light has three different bands: UV–A (320~420 nm), UV–B (275~320 nm) and UV–C (200~275 nm). By identifying suitable UV light with the specific wavelength for the flow reaction, changing the shape of the UV lamp to match the flow reactor to ensure UV light could arrive precisely where it is needed and consequently, minimize scattering loss of the UV light, which will most likely enhance the light-harvesting efficiency. Furthermore, UV light is most commonly produced by the Sun and is delivered to the Earth's atmosphere. People could optimize the converting efficiency (solar light to chemical energy) through exploring novel luminescent solar concentrators (LSC) [36]. LED lights are an eco-friendly lighting choice, since they provide relatively narrow emission bands (±20 nm) [37]. Notably, compact fluorescent light (CFL) lamps (including UV lamps) generate a lot of heat, but LED lights produce less than 10% of that. Less heat means less energy being wasted. A ballast (transformer) is not needed for LED lights, so there is less energy needed for LED lights to function. In most cases, LED lights use 40%–80% less current than fluorescent, incandescent and halogen lighting. With the least amounts of energy and hazardous materials and waste, LEDs are the best energy-efficient alternative compared to CFL lamps. Interestingly, a recent paper has shown that LED lights could act as a reaction parameter. As for the perfluoroalkylation reaction in a continuous-flow photoreactor, by screening irradiation wavelengths (16 LED arrays with different bands), the wavelengthselectivity of reactions has been revealed [38]. Undoubtedly, light sources with high-energy efficiency and with selectivity for specific reaction are vital for the study of continuous-flow photochemistry.
To avoid the potential clogging of the flow reactor, people prefer to use large amounts of solvents. This, in turn, causes the light absorption to decrease because of the decreasing of the concentration according to the Bouguer–Lambert–Beer's law (A = εlc, ε: the molar extinction coefficient; l: the path length of light propagation; c: the concentration), resulting in low-energy efficiency. Apparently, there are two factors to ensure flow-type photoreaction runs smoothly, (1) with maximum light absorption (high concentration) and (2) without reactor clogging (low concentration) should be considered simultaneously, and then a compromise way may be used. Concomitantly, insights into the relationship between the amount of solvents (concentration) and the yield of final products could be gained via research endeavours, with the aim of reducing solvents/energy waste. Takahashi and colleagues have reported that the yield of vitamin D3 from provitamin D3 through continuous-flow photolysis was quite high (HPLC-UV: 60%, isolated: 32%), and also claimed that the concentration of provitamin D3 was relatively high (30 mmol/L), namely, without high dilutions [39]. Nevertheless, this result may underline that the amount of solvents should be explored systematically, and that the effectiveness of continuous-flow photochemistry could be improved by exploiting experimental conditions.
The choice of solvent provides the basis of running photoreaction under flow type in a cost-effective, reliable and safe way. First, a simple strategy could be helpful to select the suitable solvent: measuring the UV–vis spectra of starting materials in different kinds of solvents and identifying the system with the highest overall absorbance. By doing so, the photoenergy may be harvested to the maximum extent. Second, currently, active pharmaceutical ingredients (APIs) have been manufactured by this technology [8, 40, 41]. In this regard, for the safety of the patient, appropriate selection of the solvent is primarily important because residual solvents could bring health risks. Based on the classification of United States Pharmacopoeia (http://www.usp.org), solvents are evaluated for their possible risk to human health and categorized into the three classes that follow. Class 1: solvents should be avoided, such as C6H6, CCl4, CH2ClCH2Cl and others (known human carcinogens). Class 2: solvents should be limited, such as CH3CN, C6H5Cl, CHCl3, DMF, CH3OH and others (nongenotoxic animal carcinogens or possible causative agents of other irreversible toxicity). Class 3: solvents with low toxic potential to humans, such as C2H5OH, CH3COOC2H5, CH3COCH3 and (C2H5)2O. To prompt continuous-flow photochemistry in pharmaceutical industry, the use of less toxic solvents is critical. Third, for safety concerns, attention should be paid to autoignition temperatures and flashpoints of selected solvents and, in particular, it is increasingly important when reactions are highly exothermic. The suitable solvent can lead to safe and environmentally benign processes in the flow reactor, and meet the twelve principles of green chemistry that were first articulated in 1998 [42].
If photocatalysts are being used in the flow reactions, it is imperative to consider the sustainability of photocatalysts and to maximize the potential of continuous-flow photochemistry. (1) The high stability and the excellent lifetime of photocatalysts will definitely help the subsequent scale-up and industrial processes. (2) With the superior catalytic activity, it is accessible to fabricate target molecules without harsh conditions. Chemists prefer to use mild experimental conditions, and to alleviate energy usage and reduce environmental footprint. (3) In most cases, batch processes recycle heterogeneous catalysts, such as Ru/Ir photocatalysts, and reuse them in the next run if there is no sign of deactivation. Whereas under flow type, with the deliberating reactor design, the integration of photocatalysts with flow reactors (packed bed reactors, catalytic membrane reactors and others) may bring dramatic results in terms of catalyst sustainability [43]. Certainly, this area needs input from chemical engineers.
Undoubtably, the fascinating emergent characteristics of this method are only constrained by the imagination of the chemists adopting it. In the future, it will be crucial to promote crossdisciplinary development of the potentialities among the fields of chemistry, engineering, materials science, biotechnology and even artificial intelligence (AI) [44].
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsFinancial support from the National Natural Science Foundation of China (No. 21771088), Zhejiang Provincial Natural Science Foundation of China (No. LY20B010005) and the open research funds of JLU (2020-9) & FJIRSM, CAS (No. 20170034) is gratefully acknowledged. J. Xie also appreciates Dr. Eric Wu (Corning) for his enthusiastic help
| [1] |
A. Adamo, R.L. Beingessner, M. Behnam, et al., Science 352 (2016) 61-67. DOI:10.1126/science.aaf1337 |
| [2] |
J. Zhang, C. Gong, X. Zeng, et al., Coord. Chem. Rev. 324 (2016) 39-53. DOI:10.1016/j.ccr.2016.06.011 |
| [3] |
D. Cambié, C. Bottecchia, N.J.W. Straathof, V. Hessel, T. Noël, Chem. Rev. 116 (2016) 10276-10341. DOI:10.1021/acs.chemrev.5b00707 |
| [4] |
T. Noël, J. Flow Chem. 7 (2017) 87-93. DOI:10.1556/1846.2017.00022 |
| [5] |
M. Oelgemöller, T. Goodine, P. Malakar, Flow photochemistry-a green technology with a bright future, in: L. Vaccaro (Ed.), Sustainable Flow Chemistry: Methods and Applications, Wiley-VCH, Weinheim, 2017, pp. 1-24. 10.1002/9783527689118.ch1
|
| [6] |
M. Oelgemöller, Chem. Eng. Technol. 35 (2012) 1144-1152. DOI:10.1002/ceat.201200009 |
| [7] |
M. Oelgemöller, O. Shvydkiv, Molecules 16 (2011) 7522-7550. DOI:10.3390/molecules16097522 |
| [8] |
F. Lévesque, P.H. Seeberger, Angew. Chem. Int. Ed. 51 (2012) 1706-1709. DOI:10.1002/anie.201107446 |
| [9] |
K. Loubière, M. Oelgemöller, T. Aillet, et al., Chem. Eng. Process. 104 (2016) 120-132. DOI:10.1016/j.cep.2016.02.008 |
| [10] |
D. Staveness, T.M. Sodano, K. Li, et al., Chemistry 5 (2019) 215-226. DOI:10.1016/j.chempr.2018.10.017 |
| [11] |
R.C. McAtee, E.J. McClain, C.R.J. Stephenson, Trends Analyt. Chem. 1 (2019) 111-125. DOI:10.1016/j.trechm.2019.01.008 |
| [12] |
C. Alonso, E.M. de Marigorta, G. Rubiales, et al., Chem. Rev. 115 (2015) 1847-1935. DOI:10.1021/cr500368h |
| [13] |
J.W. Beatty, J.J. Douglas, K.P. Cole, et al., Nat. Commun. 6 (2015) 1-6. |
| [14] |
K.C. Harper, E.G. Moschetta, S.V. Bordawekar, et al., ACS Cent. Sci. 5 (2019) 109-115. DOI:10.1021/acscentsci.8b00728 |
| [15] |
N. Emmanuel, C. Mendoza, M. Winter, et al., Org. Process Res. Dev. 21 (2017) 1435-1438. DOI:10.1021/acs.oprd.7b00212 |
| [16] |
I. Abdiaj, C.R. Horn, J. Alcazar, J. Org. Chem. 84 (2019) 4748-4753. DOI:10.1021/acs.joc.8b02358 |
| [17] |
M. Sayes, G. Benoit, A.B. Charette, Angew. Chem. Int. Ed. 57 (2018) 13514-13518. DOI:10.1002/anie.201807347 |
| [18] |
M. Hunger, G. Hüsken, H.J.H. Brouwers, Concrete Res. 40 (2010) 313-320. DOI:10.1016/j.cemconres.2009.09.013 |
| [19] |
J. Mo, Y. Zhang, Q. Xu, et al., Atmos. Environ. 43 (2009) 2229-2246. DOI:10.1016/j.atmosenv.2009.01.034 |
| [20] |
S. Wang, H.M. Ang, M.O. Tade, Environ. Int. 33 (2007) 694-705. DOI:10.1016/j.envint.2007.02.011 |
| [21] |
J. Mo, Y. Zhang, R. Yang, et al., Build. Environ. 43 (2008) 238-245. DOI:10.1016/j.buildenv.2005.12.027 |
| [22] |
Q.L. Yu, H.J.H. Brouwers, Appl. Catal. B -Environ. 92 (2009) 454-461. DOI:10.1016/j.apcatb.2009.09.004 |
| [23] |
L. Yang, Z. Liu, J. Shi, et al., Catal. Today 126 (2007) 359-368. DOI:10.1016/j.cattod.2007.06.017 |
| [24] |
F.B. Li, X.Z. Li, C.H. Ao, et al., Chemosphere 59 (2005) 787-800. DOI:10.1016/j.chemosphere.2004.11.019 |
| [25] |
N. Wang, X. Zhang, Y. Wang, et al., Lab Chip. 14 (2014) 1074-1082. DOI:10.1039/C3LC51233A |
| [26] |
S. Malato, P. Fernández-Ibánez, M.I. Maldonado, et al., Catal. Today 147 (2009) 1-59. DOI:10.1016/j.cattod.2009.06.018 |
| [27] |
L. Lei, N. Wang, X.M. Zhang, et al., Biomicrofluidics 4 (2010) 043004. DOI:10.1063/1.3491471 |
| [28] |
M. Rahimi, B. Aghel, M. Sadeghi, et al., Water Treat. 52 (2014) 5513-5519. DOI:10.1080/19443994.2013.807471 |
| [29] |
S.P. Albu, A. Ghicov, J.M. Macak, et al., Nano Lett. 7 (2007) 1286-1289. DOI:10.1021/nl070264k |
| [30] |
L. Wang, A. Mazare, I. Hwang, et al., Chem. Commun. 53 (2017) 11763-11766. DOI:10.1039/C7CC06889A |
| [31] |
H. Seo, M.H. Katcher, T.F. Jamison, Nat. Chem. 9 (2017) 453-456. DOI:10.1038/nchem.2690 |
| [32] |
H. Seo, A. Liu, T.F. Jamison, J. Am. Chem. Soc. 139 (2017) 13969-13972. DOI:10.1021/jacs.7b05942 |
| [33] |
D. Cambié, F. Zhao, V. Hessel, et al., Angew. Chem. Int. Ed. 56 (2017) 1050-1054. DOI:10.1002/anie.201611101 |
| [34] |
J.A. Lv, Y. Liu, J. Wei, et al., Nature 537 (2016) 179-184. DOI:10.1038/nature19344 |
| [35] |
N.K. Shrestha, J.M. Macak, F. Schmidt-Stein, et al., Angew. Chem. Int. Ed. 48 (2009) 969-972. DOI:10.1002/anie.200804429 |
| [36] |
D. Cambié, J. Dobbelaar, P. Riente, et al., Angew. Chem. Int. Ed. 58 (2019) 14374-14378. DOI:10.1002/anie.201908553 |
| [37] |
L. Buzzetti, G.E.M. Crisenza, P. Melchiorre, Angew. Chem. Int. Ed. 58 (2019) 3730-3747. DOI:10.1002/anie.201809984 |
| [38] |
C.P. Haas, T. Roider, R.W. Hoffmann, et al., React. Chem. Eng. 4 (2019) 1912-1916. DOI:10.1039/C9RE00339H |
| [39] |
S. Fuse, N. Tanabe, M. Yoshida, et al., Chem. Commun. 46 (2010) 8722-8724. DOI:10.1039/c0cc02239j |
| [40] |
J. Turconi, F. Griolet, R. Guevel, et al., Org. Process Res. Dev. 18 (2014) 417-422. DOI:10.1021/op4003196 |
| [41] |
J. Turconi, F. Griolet, R. Guevel, et al., Org. Process Res. Dev. 18 (2014) 831. DOI:10.1021/op5001397 |
| [42] |
J.H. Clark, Green Chem. 1 (1999) 1-8. DOI:10.1039/a807961g |
| [43] |
A. Gavriilidis, A. Constantinou, K. Hellgardt, et al., React. Chem. Eng. 1 (2016) 595-612. DOI:10.1039/C6RE00155F |
| [44] |
C.W. Coley, D.A. Thomas Ⅲ, J.A.M. Lummiss, et al., Science 365 (2019) eaax1566. DOI:10.1126/science.aax1566 |
 2020, Vol. 31
2020, Vol. 31 



