Metal organic framework (MOF) is a new type of porous crystal material [1]. Recently, owing to its high specific surface area and porosity, diversity of metal centers and ligands, and structure, MOF has been extensively studied in energy storage, catalysis, gas adsorption and separation, and chemical sensors [2-4]. More importantly, in order to effectively apply MOF materials, MOF is used as a sacrificial template to derive metal oxides, metal sulfides, metal phosphates, non-metal heteroatom doped derivatives and other porous nanomaterials [5-7]. These materials can provide high electrical conductivity and electrochemical reactivity [8]. For example, NiCo2O4 and NiCo2S4 nanoparticles with mesoporous polyhedron structure successfully prepared by using bimetallic zeolitic imidazole frameworks as precursors have ultra-high electrochemical performance as electrodes of supercapacitors [9]. A report confirms that the Ni-Fe-P nanosheets obtained by direct phosphating of MOF precursors have good electrocatalytic activity for hydrogen/oxygen evolution reaction [10]. In addition, Fe1-xS/N, S co-doped carbon composites synthesized by simultaneously decomposing and sulfurizing Fe-MOF can further improve the storage capacity of lithium ion [11]. Therefore, these successful cases confirm that MOF-derived materials exhibit a very promising futrue in various application.
As a kind of energy storage equipment, supercapacitors have unique advantages in electrochemical applications: high power density, long cycle life and charge-discharge rate. MOF-derived metal oxides are a common electrode material for supercapacitors [12]. Due to the incomplete reversible redox reaction, the pseudo electric container may lack cycle stability and rate performance, resulting in low energy density [13]. In order to increase the energy density, in addition to increasing the specific surface area, another method is to dope the non-metallic heteroatoms such as nitrogen, phosphorus, sulfur, etc into metal oxides [14]. This kind of nonmetallic heteroatom doped materials can be obtained by the direct pyrolysis of MOF containing non-metallic heteroatoms, or introducing into the samples through the pyrolysis process in different atmospheres [15, 16]. Of these dopants, nitrogen seems to be the most effective dopant because it is similar in size to oxygen and has less ionization energy [17]. On the one hand, the incorporation of nitrogen can significantly enhance the total capacitance through the pseudo-capacitance effect [18]. On the other hand, it can change the electronic properties, provide more active sites to boost the conductivity of metal oxides and enhance the interaction between active materials and electrolytes, so as to improve the kinetics of ion diffusion and transfer [19]. At the early stage, nitrogen doped TiO2 has been studied mostly, and a lot of efforts have been made in its synthesis, characterization and application [20-23]. The next focus is mainly on doping some other metal oxides such as ZnO, Co3O4, MnO2 with nitrogen. Related reports display that the use of a controlled nitrogen-doping strategy can notably raise the catalytic activity of Co3O4 on oxygen evolution/reduction reaction [24], and the nitrogen-doped Co3O4 nanowires and nitrogen-doped ZnO nanorods researched by Huang et al. have been proved to be suitable for the cathode materials of flexible solid zinc air batteries and Zn-MnO2 batteries, respectively, indicating high volume capacity and better cycle stability [25, 26]. In addition, spinel bimetallic oxides have been testified to have high electrochemical activity. Nitrogen-doped CuCo2O4@C composite was prepared by Jiao et al. successfully. Benefiting from the synergistic effect of ultra-small CuCo2O4 nanoparticles and customized nitrogen-doped carbon matrix, the composite exhibits high cycle stability and rate capacity [27].
So far, in addition to CuCo2O4, spinel bimetal oxides has been reported on NiCo2O4 [28], ZnCo2O4 [29] and MnCo2O4 [30], which shows high capacity and stability. Therefore, such metal oxides are considered promising electrode materials because they can effectively overcome the shortcomings of simple oxides and combine two types of functional materials for the synergistic effect to enhance the intrinsic properties of each component including electrochemical reactions and mechanical stability [31]. In particular, nickel cobalt oxides (NiCoO) have been widely studied as electrode materials for supercapacitors in recent years [32]. Because of the combination of nickel and cobalt, the NiCoO have a richer redox reaction when used as electrode materials, and the lower electron transfer activation energy makes them usually have higher electrical conductivity than single nickel and cobalt oxide, and exhibit better electrochemical property [33, 34]. NiCoO is isomorphic to Co3O4 crystal structure, in which the Co2+ is partially replaced by Ni2+ [35]. Since both Ni and Co exhibit capacitive behavior, it is necessary to explore and design a appropriate ratio between Ni and Co to obtain good electrochemical performance [36]. Several reports of NiCoO applied to supercapacitors reveal that the product with Ni/Co = 1:1 still achieved the best performance [37, 38]. On the other hand, it is worth noting that porous nanostructure and high specific surface area can accelerate the electron transfer and electrolyte diffusion at the electrode-electrolyte interface to further improve the electrochemical performance [39]. Two similar reports have successfully synthesized hierarchical porous NiCo2O4 and obtained excellent performance in the application of supercapacitors [40, 41].
Inspired by the previous work, considering the application of nitrogen-doped composite metal oxides to supercapacitors electrode materials is promising. In this work, we propose a very simple direct pyrolysis method at high temperature. The precursors of metal-organic framework (denoted by Co-MOF, Ni-Co-MOF-n, Ni-MOF, n represents the molar ratio of Ni to Co, n = 1, 2, 4) were reasonably synthesized by using different ratios of Ni to Co and organic nitrogen-containing ligands, and then five kinds of nitrogen doped metal oxides (denoted by N-Co3O4, N-NiCoO-n and N-NiO) with different Ni/Co molar ratios and good hexagonal morphology are derived from these corresponding precursors. In addition, electrochemical test results indicate that all N-NiCoO samples have higher capacitance than that of single nickel and cobalt oxides. Moreover, N-NiCoO-2 with a Ni/Co metal ratio of 2:1 displays the highest capacitance of 945.79 F/g and excellent cycle stability. This superior capacitive behavior is mainly attributed to: N-NiCoO has better conductivity and has multiple metal oxidation states, which can produce many kinds of redox reactions as electrode materials. Then, doping nitrogen can provide more active sites for NiCoO to heighten its conductivity and enhance the interaction between the electrode material and the electrolyte. Finally, N-NiCoO is porous and has a stable hexagonal structure, which can accelerate the electron transfer at the interface and promote the electrolyte diffusion and OH- insertion/desorption.
Synthesis of Co-MOF: Co(NO3)2·6H2O (0.4365 g, 1.5 mmol), H3BTC (H3BTC = 1, 3, 5-benzenetricarboxylic acid, 0.3165 g, 1.5 mmol), and 4, 40-bipy (4, 40-bipy = 4, 40-bipyridine, 0.288 g, 1.5 mmol) are dissolved in 60 mL DMF (DMF = N, N-dimethylformamide), then sealed in 100 mLTeflon-lined autoclave andkeptat353 Kfor18 h preparedby a solvothermal synthesis route. When cooled down to room temperature, the product is washed thoroughly with DMF and ethanol three times to remove impurities and dry in air naturally.
Synthesis of Ni-MOF: Ni-MOF is synthesized using a similar procedure to that described above, except for the use of Ni(NO3)2· 6H2O (0.4362 g, 1.5 mmol) as the metal source.
Synthesis of Ni-Co-MOF-n: The precursors with various ratios of Ni(NO3)2·6H2O to Co(NO3)2·6H2O are readily synthesized as described above, which are designated as Ni-Co-MOF-n (n represents the molar ratio of Ni(NO3)2·6H2O to Co(NO3)2· 6H2O, n = 1, 2, 4).
Synthesis of N-metal oxide materials: Co-MOF, Ni-Co-MOF-n and Ni-MOF are introduced into a quartz crucible placed within a horizon quartz tube furnace at a ramp rate of 1 ℃/min to final temperature of 450 ℃ in air. After thermo-decomposition of the organic species, the sample is slowly cooled to room temperature. The resulting samples obtained are nitrogen doped nickel oxides, nitrogen doped nickel-cobalt oxides and nitrogen doped cobalt oxides (denoted as N-Co3O4, N-NiCoO-n, and N-NiO), which are derived from Co-MOF, Ni-Co-MOF-n, and Ni -MOF, respectively.
The morphological features are characterizedby scanning electron microscopy (SEM, Zeiss-Supra 55), high resolution transmission electron microscopy (HRTEM, Tecnai G2 F30 S-TWIN) and energy dispersive spectroscopy (EDS) mapping. X-ray diffraction (XRD) patterns are examined on a Bruker D8 Advanced X-ray diffractometer (CuKα radiation:λ = 0.15406 nm). The chemical states are measured using an Axis Ultra X-ray photoelectron spectroscope (XPS, Kratos Analytical Ltd., UK) equippedwith a standard monochromaticAl-Kα source (hv = 1486.6 eV). In addition, N2 adsorption-desorption measurements are performed on Quantachrome Instruments, Autosorb IQ3. The thermogravimetric analysis (TGA) is performed under airatmosphere with a heating rateof 5 ℃/minbyusing aPyris 1 TGA thermogravimetric analyzer.
The electrochemical measurements are carried outwith CHI660e working station in 3.0 mol/L KOH solution at room temperature. A Hg-HgO electrode and platinum electrode are chosen as reference and counter electrode, respectively. Galvanostatic charge-discharge (GCD), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) methods are used to investigate the capacitive propertiesof the N-Co3O4, N-NiCoO-n, and N-NiO electrodes. The EIS measurements are conducted in the frequency range of 100 kHz to 0.01 Hz at the open-circuit voltage.
For the three-electrode cell, the working electrode is made by mixing the active materials samples (N-Co3O4, N-NiCoO-n, and N-NiO), acetylene black and polytetrafluoroethylene at a weight ratio of 80:15:5 respectively, and coating the mixture on a 1 cm × 5 cm nickel foam. The additive is a certain amount of isopropyl alcohol when grinding and the painted size is about 1 cm2, which is then pressed into a thin foil at a pressure of 10.0 MPa. The typical mass loading of the electrode material is 1.0 mg.
When assembling an aqueous device, samples are employed as positive electrode while negative electrode is activated carbon. The positive and negative electrodes are made by mixing samples (N-Co3O4, N-NiCoO-n and N-NiO)/activated carbon, acetylene black and polytetrauoroethylene at a weight ratio of 80:15:5 respectively. The slurry is coated on a piece of foamed nickel foam (≈ 1 cm2), which is then pressed into a thin foil at a pressure of 10 MPa. The mass ratio of the positive/negative electrode active material is 1:4, and electrochemical performance is measured in an aqueous device at room temperature.
Firstly, five precursors (Co-MOF, Ni-Co-MOF-n, Ni-MOF) with different Ni/Co molar ratios are successfully synthesized. The morphology and composition of these precursors are characterized by scanning electron microscopy (SEM) and X-ray diffraction (XRD), as shown in Figs. S1a-e and S2 (Supporting information). SEM pictures exhibit that Co-MOF is a kind of nanorod structure, and Ni-Co-MOF-n and Ni-MOF are typical regular hexagonal nanoplates with a certain thickness. Observing the scale in the figure, it is found that the diagonal of the Ni-Co-MOF-n regular hexagonal nanoplates are longer, about three times as long as that of Ni-MOF. However, compared to the thickness of the two, the Ni-Co-MOF-n regular hexagonal nanoplates are thinner. The obtained results from XRD patterns (Fig. S2) are consistent with the standard pattern [12], indicating that the five precursors are the materials with the same crystal structure. After that, in order to obtain a suitable calcination temperature, the dried precursor samples are subjected to thermogravimetric analysis at 0800 ℃ in air. From Fig. S3 (Supporting information), all precursors possess similar thermogravimetric curves. At temperatures below 250 ℃, the weight loss is caused by the escape of adsorbed water and solvent molecules. The subsequent significant weight loss at 250-400 ℃ is attributed to oxidation of the ligands and decomposition of the skeleton. The crystallization of metal oxides takes place almost after 400 ℃. At higher temperatures, no significant weight loss is observed, indicating that there are no other phase or structural changes in the formedmetal oxide.Based on the above change process, 450 ℃is selected as the calcination temperature to ensure that the precursors are completely converted into oxidation products.
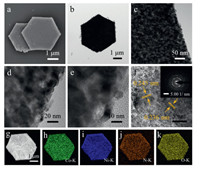
The oxidation derivatives are obtained from the precursors in air at 450 ℃. In rapid sequence, the oxidation products with different Ni/Co ratios are examined for morphological characteristics by SEM and transmission electron microscopy (TEM). It can be seen from the SEM images (Fig. 1a and Figs. S4-S7 in Supporting information) that due to the decomposition of the skeleton, the shapes of N-Co3O4, N-NiCoO-n, and N-NiO are rough and the size is reduced. But even after heat treatment, they still retain the morphology of the MOF precursors: long nanorods and hexagonal nanoplates structure, showing excellent structural stability. To ulteriorly understand morphology and microstructure of derived nitrogen doped metal oxides, TEM analysis is carried out. At high magnification, TEM images (Fig. 1b-e and Figs. S4-S7) present that long nanorods and hexagonal nanoplates are composed of many small nanoparticles, among N-NiCoO-2 particles are denser. In addition, the porous characteristics can be conducive to the diffusion of electrolyte solution in the electrochemical test process. The lattice fringe obtained in the high-resolution TEM (HRTEM) (Fig. 1f and Figs. S4-S7) images display distance between N-Co3O4 and N-NiO match well with the corresponding crystal faces. The interplanar spacing of the three N-NiCoO-n samples prepared in Fig. 1, Figs. S5 and S6 is ≈ 0.287, 0.245, 0.236 and 0.201 nm, respectively, representing the (220), (311), (222) and (400) lattice planes of the spinel NixCo3-xO4. Morever, selected area electron diffraction (SAED) patterns of all the obtained nitrogendoped metal oxides attest that they have polycrystalline features. The energy dispersive X-ray spectroscopy (EDS) mapping images in Figs. 1g-k and Figs. S4-S7 determine the coexistence and uniform distribution of Co, N and O in N-Co3O4, Ni, N and O in N-NiO, and Ni, Co, N and O in three N-NiCoO-n samples. Further EDS quantitative analysis results (Fig. S8 in Supporting information) confirm that the Ni/Co molar ratios of the five samples are approximately 0:1, 1:1, 2:1, 4:1 and 1:0, respectively. In comparison, N-NiCoO-2 has the most nitrogen content.
|
Download:
|
| Fig. 1. (a) SEM image, (b-e) TEM images, (f) HRTEM image and SAED pattern of NNiCoO-2. (g-k) EDS-mapping images of (h) Ni; (i) Co; (j) N; (k) O for N-NiCoO-2. | |
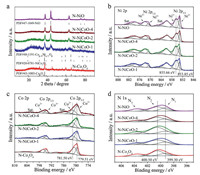
The crystalline phase and chemical composition of calcined powder samples are identified by XRD. The results prove that the precursor is successfully converted into the desired oxidation products. As shown in Fig. 2a, Co-MOF and Ni-MOF are converted into pure spinel Co3O4 and NiO, respectively, which corresponds to the standard patterns (PDF#43-1003 and PDF#47-1049) well. Furthermore, the NiCoO-n products obtained by corresponding Ni-Co-MOF-n match the standard pattern of NiCo2O4 and Co1.29Ni1.71O4 with spinel structure (PDF#20-0781 and PDF#40- 1191), and almost no other phases or impurities are detected. Pure NiO can be observed in NiCoO-n, which indicates that Ni atoms have been well integrated into Co3O4 lattice. The results are consistent with the SAED. To investigate more detailed elemental composition and valence of obtained N-Co3O4, N-NiCoO-n and N-NiO products, X-ray photoelectron (XPS) measurements are conducted. The survey spectrums in Fig. S9 (Supporting information) once again substantiate the subsistence of Ni, Co, C, N and O in these products. Ni 2p spectrums comprise two main peaks including Ni 2p3/2 and Ni 2p1/2. Fitting peaks at 853.85 and 871.09 eV correspond to Ni2+, while those at 855.66 and 872.88 eV correspond to Ni3+ (Fig. 2b) [34]. In these four spectra, the amount of Ni3+ is higher than Ni2+, which can form the active redox center Ni2+/Ni3+, and with the increase of Ni content, the Ni2+ fitting peak intensity of 2p3/2 increases. It is possible that the formation of NiO gradually exhausts the Co positions on the surface of N-NiCoO-n hexagonal nanoplates [42]. Similarly, the Co 2p spectrums in Fig. 2c consist of two spin orbit double peaks with the characteristics of Co2+ (781.50 eV for 2p3/2, 796.67 eV for 2p1/2) and Co3+ (779.51 eV for 2p3/2, 794.78 eV for 2p1/2) and two satellite peaks [43]. As the Ni content increases, the Co2+ fitting peaks intensity of 2p3/2 decreases. The O 1s spectrums (Fig. S10 in Supporting information) show three different oxygen characteristics. Specifically, the binding energies of the three fitting peaks are 529.88 eV, 531.21 eV and 532.70 eV respectively, which can be attributed to metal oxygen (O1), oxygen defect (O2) and chemisorption oxygen (O3) [44, 45]. The N 1s spectrums (Fig. 2d) can also be divided into three fitting peaks centered on the binding energy of 399.30, 400.50 and 403.60 eV, corresponding to pyridine nitrogen (N1), pyrrole (N2) and Pyridine-N-oxide (N3), respectively [46]. It is reported that the combination of pyridine nitrogen (N1) and pyrrole nitrogen (N2) contributes to better pseudocapacitors [14]. In order to obtain the specific surface area of the samples, the porous texture of N-Co3O4, N-NiCoO-n and N-NiO is indagated by N2 adsorption-desorption isotherms. The specific surface area values for all samples are summarized in Table S1 (Supporting information). As shown in Fig. S11 (Supporting information), the isotherms are all of type IV curves with hysteresis loops at a relative pressure of 0.8–1.0, meaning that there are a large number of mesopores in nitrogen-doped metal oxides. The pore size distribution (Fig. S12 in Supporting information) for the BarrettJoyner-Halenda adsorption branch of five products further displays sharp peaks was observed around 3.50 nm and a broad peak begin to appear around 20 nm. These results clearly indicate the presence of micro, mesopores and even macropores in the products.
|
Download:
|
| Fig. 2. a) XRD patterns and (b) Ni 2p spectra of N-NiCoO-1, N-NiCoO-2, N-NiCoO-4 and N-NiO, (c) Co 2p spectra of N-Co3O4, N-NiCoO-1, N-NiCoO-2, N-NiCoO-4. (d) N 1s spectra of N-Co3O4, N-NiCoO-1, N-NiCoO-2, N-NiCoO-4 and N-NiO. | |
In a three-electrode test system, the electrochemical performance of bare Ni-foam electrode was evaluated via exploiting Pt as counter electrode and Hg-HgO as reference electrode. As shown in Fig. S13 (Supporting information), the bare electrode is charged and discharged rapidly under the voltage window of 0.49 V. At the current density of 1 A/g, the specific capacitance is only 15.10 F/g. The synthesized rod-shaped N-Co3O4 and hexagonal nanoplateshaped N-NiCoO-n and N-NiO are prepared as electrodes to perform a suite of electrochemical properties testings in a threeelectrode system. Figs. S14 and S15 (Supporting information) exhibit the cyclic voltammogram (CV) curves of five electrodes completed in 3.0 mol/L KOH electrolyte at different potentials and scanning rates. The CV curves are composed of obvious redox peaks, which are mainly derived from the Faraday pseudocapacitive behavior provided by Ni and Co ions in the electrode material. This is almost different from the typical electric double layer CV curves in rectangle, as well as confirming the previous XPS results. In addition, comparing the CV curves of the N-Co3O4, N-NiCoO-n and N-NiO electrodes at 20 mV/s in Fig. 3a, it is found that when Ni/Co molar ratio reaches 2, the area around the CV curve of N-NiCoO-2 electrode is larger than that of other electrodes, indicating this electrode exhibits the largest charge storage capacity among five electrodes. Through the observation of all CV curves, only one pair of redox peaks appeared in the CV curves of N-Co3O4 and N-NiO electrodes, while the CV curves of NNiCoO-n appeare to have two pairs of redox peaks, and the most obvious one is N-NiCoO-2, and the peak strength gradually weakened with the increase of Ni/Co ratio. These redox peaks can be described as a two-phase reversible electrochemical reaction mechanism [32, 47]:
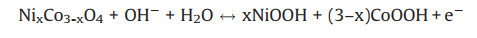
|
(1) |

|
(2) |

|
(3) |
|
Download:
|
| Fig. 3. Electrochemical results of as-prepared electrodes (N-Co3O4, N-NiCoO-1, N-NiCoO-2, N-NiCoO-4 and N-NiO) in a three-electrode cell in 3.0 mol/L KOH aqueous solution: (a) CV curves with a scan rate at 20 mV/s. (b) GCD curves at a current density 1 A/g. (c) Specific capacitance at different current densities. (d) Cycling performance at 5 A/g for 5, 000 cycles. | |
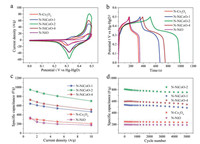
In order to further evaluate the electrochemical performance of the prepared N-Co3O4, N-NiCoO-n and N-NiO, Galvanostatic charge-discharge (GCD) curves under varied current densities are shown in Fig. S16 (Supporting information). The symmetry of GCD curves is greet and the plateau region is consistent with the peak observed in CV curves, meaning that the electrode material has excellent pseudo capacitance characteristics. At 1 A/g, NNiCoO-2 presents the longest charge discharge time (Fig. 3b). Ground on GCD curves, the homologous specific capacitance is computed in Fig. 3c. Specific capacitance of N-NiCoO-2 (945.79 F/g) is much higher than that of N-NiCoO-1 (641.40 F/g), N-NiCoO-4 (725.11 F/g), N-Co3O4 (316.35 F/g), N-NiO (336.15 F/g) at 1 A/g, and other similar materials. But as the current density increases, the capacitance will be lost. Fortunately, when the current density is increased by 10 times (10 A/g), N-NiCoO-2 can still maintain a capacitance of 700.00 F/g. These results indicate that when the Co portion of Co3O4 is replaced by Ni, the corresponding capacitance also increases appropriately. When a certain ratio is reached (Ni:Co = 2:1), the highest specific capacitance can be obtained. When Ni completely replaces Co, the specific capacitance is greatly reduced. Therefore, the addition of a certainpercentage of Ni enables the metal oxide to more effectively exert redox properties, enhancing the electrical conductivity to improve electrochemical performance. In addition, doping nitrogen provides more active sites, and enhanced pseudocapacitance makes N-NiCoO-2 with high specific surface area and nitrogen contentoutstanding. After5000 cycles, the specific capacitance of N-NiCoO-2 can still keep at 93.30% of the original value, while the other electrodes maintain at 90.10% (NNiCoO-1), 91.20% (N-NiCoO-4), 95.00% (N-Co3O4), 96.50% (N-NiO) (Fig. 3d). A comparison between the superior performance of the nitrogen-doped hexagonal NiCoO nanoplates and other similar materials is given in Table S2 (Supporting information). The electrochemical characteristics of the electrodes are evaluated by electrochemical impedance spectroscopy in Fig. S17 (Supporting information). It can be see that the N-NiCoO-n electrodes show a smaller resistance than the N-Co3O4 and N-NiO, indicating a higher conductivity.
Activated carbon (AC) is an excellent anode material with good conductivity, low cost and electrochemical stability. For the sake of further indagating the nitrogen-doped metal oxide electrodes in practical application, N-Co3O4, N-NiCoO-n, N-NiO are used as positive electrode, and AC is used as negative electrode, and 3.0 mol/L KOH solution is used as electrolyte. In two-electrode devices, the working potential range is extended to 0–1.2 V (Figs. S18 and S19 in Supporting information). Observing that the CV curves of N-Co3O4//AC, N-NiCoO-n//AC and N-NiO//AC display similar redox trend to the three electrode system, and N-NiCoO-2//AC presents stronger redox peak and larger CV curve area in Fig. 4a. However, the CV curves still keep a good shape at high sweep speed, indicating excellent rate performance. The specific capacitance of all aqueous devices are calculated by GCD curves under varied current densities (Fig. 4b and Fig. S20 in Supporting information) from 1 A/g to 10 A/g. As shown in Fig. 4c, specific capacitance of N-NiCoO-2//AC device can reach 260.59 F/g at 1 A/g, much higher than that of N-NiCoO- 1//AC (202.95 F/g), N-NiCoO-4//AC (215.08 F/g), Co3O4//AC (103.00 F/g) and NiO//AC (107.50 F/g), and it still keep the value of 215.47 F/g at 10 A/g, which benefits from nitrogen doping properties and good conductivity of N-NiCoO-2. In addition, the charge-discharge cycle test is performed at 5 A/g. After 5, 000 cycles, N-NiCoO-2//AC still maintains 90.00% of the original specific capacitance, which displays its excellent cycle stability (Fig. 4d). Good electrochemical performance can also be confirmed by the lower Rct of the N-NiCoO-2//AC device (Fig. S21 in Supporting information). With the view of exploring total properties of N-NiCoO-n//AC aqueous devices, Ragone diagram is drawn from the GCD curves found on its twoelectrode system in Fig. 4e. Energy density of N-NiCoO-2//AC can reach 52.12 Wh/kg at the power density of 600.00 W/kg, while it can still remain 43.09 Wh/kg at the high power density of 5775.82 W/kg, which is significantly higher than other four devices. Meanwhile, the N-NiCoO-2//AC device performs well compared to the similar materials that have been reported recently (Fig. S22 in Supporting information), e.g., flower-like Ni1.5Co1.5O4//AC (47.50 Wh/kg at 855.00 W/kg) [36], NixCo3-xO4/ CNTs//AC (23.56 Wh/kg at 800.15 W/kg) [48], NiCo2O4 nanowires//AC (22.60 Wh/kg at 219.00 W/kg) [40] and CC@NiCo2O4// AC (31.90 Wh/kg at 2900.00 W/kg) [28].

|
Download:
|
| Fig. 4. Electrochemical characterization of the as-prepared aqueous devices: (a) CV curves with a scan rate of 20 mV/s. (b) GCD curves at a current density of 1 A/g. (c) Specific capacitance at different current densities. (d) Cycling performance at 5 A/g for 5000 cycles. (e) Ragone plots exhibiting the relationship between energy density and power density. | |
In a word, the precursors obtained by simple solvothermal method is successfully calcined to prepare nitrogen doped oxides with different Ni/Co molar ratios. The results reveal that the size of N-NiCoO is smaller than that of pure Co3O4 and NiO, making the better diffusion of electrolyte ions in the electrode, and their rich oxidation states lead to high conductivity. Therefore, N-NiCoO performs well in the electrochemical tests of three-electrode systems and aqueous devices. Among them, N-NiCoO-2 with Ni/Co molar ratio of 2:1 stands out. At 1 A/g, the capacitance is as high as 945.79 F/g, and exhibits high cycle stability after 5000 cycles with only 6.7% attenuation at 5 A/g. The addition of a certain proportion of Ni makes the metal oxides have a lower activation energy of electron transfer, which plays a more effective role in redox properties to redound electrical conductivity and then improve electrochemical performance. More importantly, N-NiCoO-2 has a relatively high nitrogen content. The incorporation of nitrogen enhances the electron-donating ability of the metal oxide and provides more nitrogen-containing active sites, promoting the interaction between the electrode material and the electrolyte. In addition, the unique hexagonal nanoplates structure, high specific surface area and high porosity of N-NiCoO are also one of the important reasons for its outstanding electrochemical behavior. Considering the simple synthesis method, simple structure and excellent performance, this subject material may be a promising electrode material for supercapacitors.
Declaration of competing interestsThe authors declare that there are no conflicts of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (NSFC, Nos. 21671170, 21673203, 21201010 and U1904215), the Top-notch Academic Programs Projectof Jiangsu Higher Education Institutions (TAPP), Program for New Century Excellent Talents of the University in China (NCET, No.13-0645), the Six Talent Plan (No. 2015-XCL-030) and Qinglan Project. We also acknowledge the Priority Academic Program Development of Jiangsu Higher Education Institutions and the technical support we received at the Testing Center of Yangzhou University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.027.
| [1] |
(a) Y. Xu, Q. Li, H. Xue, H. Pang, Coord. Chem. Rev. 376(2018) 292-318; (b) C.S. Liu, J. Li, H. Pang, Coord. Chem. Rev. 410(2020) 213222. |
| [2] |
(a) L. Wang, Y. Han, X. Feng, et al., Coord. Chem. Rev. 307(2016) 361-381; (b) Y. Wang, Y. Wang, L. Zhang, C. Liu, H. Pang, Inorg. Chem. Front. 6(2019) 2514-2520. |
| [3] |
(a) X. Zhang, L. Chang, Z. Yang, et al., Nano Res. 12(2019) 437-440; (b) D.M. Chen, J.Y. Tian, M. Chen, C.S. Liu, M. Du, ACS Appl. Mater. Inter. 8(2016) 18043-18050. |
| [4] |
(a) X. Li, X. Yang, H. Xue, H. Pang, Q. Xu, Energy Chem. 2(2020)100027; (b) D.M. Chen, N.N. Zhang, C.S. Liu, M. Du, ACS Appl. Mater. Interfaces 9(2017) 24671-24677. |
| [5] |
L. Jin, X. Li, C. Liu, H. Pang, Chin. Chem. Lett. 30 (2019) 10-13. |
| [6] |
Y. Wang, C. Wang, Y. Wang, H. Liu, Z. Huang, J. Mater. Chem. A 4 (2016) 5428-5435. DOI:10.1039/C6TA00236F |
| [7] |
(a) B. You, N. Jiang, M. Sheng, et al., Chem. Mater. 27(2015) 7636-7642; (b) J. Ma, F. Ren, G. Wang, et al., Int. J. Hydrogen Energy 42(2017) 11654-11661. |
| [8] |
Y. Li, Y. Xu, W. Yang, et al., Small 14 (2018) 1704435. DOI:10.1002/smll.201704435 |
| [9] |
Y. Liu, Z. Wang, Y. Zhong, et al., Adv. Funct. Mater. 27 (2017) 1701229. DOI:10.1002/adfm.201701229 |
| [10] |
C. Gu, J. Li, G. Yang, et al., Chin. Chem. Lett. (2020). DOI:10.1016/j.cclet.2020.02.044 |
| [11] |
Y. Liu, H. Wang, K. Yang, et al., Appl. Sci. Basel 9 (2019) 2677. |
| [12] |
(a) Y. Li, Y. Xu, Y. Liu, H. Pang, Small 15(2019) 1902463; (b) M. Du, M. Chen, X.G. Yang, et al., J. Mater. Chem. A. 2(2014) 9828-9834. |
| [13] |
B. Xu, H. Duan, M. Chu, G. Cao, Y. Yang, J. Mater. Chem. A 1 (2013) 4565. DOI:10.1039/c3ta01637d |
| [14] |
Y. Deng, Y. Xie, K. Zou, X. Ji, J. Mater. Chem. A 4 (2016) 1144-1173. DOI:10.1039/C5TA08620E |
| [15] |
Y. Mao, H. Duan, B. Xu, et al., Energy Environ. Sci. 5 (2012) 7950. DOI:10.1039/c2ee21817h |
| [16] |
(a) S. Chao, H. Wu, Q. Xia, G. Wang, Chem Electro Chem 6(2019) 3940-3948; (b) T. Wang, X. Guo, H. Duan, C. Chen, H. Pang, Chin. Chem. Lett. 31(2020) 654-666. |
| [17] |
X. Qiu, C. Burda, Chem. Phys. 339 (2007) 1-10. DOI:10.1016/j.chemphys.2007.06.039 |
| [18] |
L. Zhao, Y. Hu, H. Li, Z. Wang, L. Chen, Adv. Mater. 23 (2011) 1385-1388. DOI:10.1002/adma.201003294 |
| [19] |
Y. Fu, Y. Huang, Z. Xiang, G. Liu, D. Cao, Eur. J. Inorg. Chem. 2016 (2016) 2100-2105. DOI:10.1002/ejic.201500822 |
| [20] |
H. Tian, L. Hu, C. Zhang, et al., J. Phys. Chem. C 114 (2010) 1627-1632. DOI:10.1021/jp9103646 |
| [21] |
(a) R. Asahi, T. Morikawa, H. Irie, T. Ohwaki, Chem. Rev.114(2014) 9824-9852; (b) K. Huang, B. Li, M. Zhao, et al., Chin. Chem. Lett. 28(2017) 2195-2206. |
| [22] |
Y. Wu, X. Liu, Z. Yang, L. Gu, Y. Yu, Small 12 (2016) 3522-3529. DOI:10.1002/smll.201600606 |
| [23] |
H. Zeng, B. Xing, L. Chen, et al., Nanomaterials 9 (2019) 1253. DOI:10.3390/nano9091253 |
| [24] |
X. Li, J. Wei, Q. Li, et al., Adv. Funct. Mater. 28 (2018) 1800886. DOI:10.1002/adfm.201800886 |
| [25] |
M. Yu, Z. Wang, C. Hou, et al., Adv. Mater. 29 (2017) 1602868. DOI:10.1002/adma.201602868 |
| [26] |
Y. Huang, W. He, P. Zhang, X. Lu, Funct. Mater. Lett. 11 (2018) 1840006. DOI:10.1142/S1793604718400064 |
| [27] |
X. Wang, K. Cao, Y. Wang, L. Jiao, Small 13 (2017) 1700873. DOI:10.1002/smll.201700873 |
| [28] |
C. Guan, X. Liu, W. Ren, et al., Adv. Energy Mater. 7 (2017) 1602391. DOI:10.1002/aenm.201602391 |
| [29] |
H. Wu, Z. Lou, H. Yang, G. Shen, Nanoscale 7 (2015) 1921-1926. DOI:10.1039/C4NR06336H |
| [30] |
S. Sahoo, K.K. Naik, C.S. Rout, Nanotechnology 26 (2015) 455401. DOI:10.1088/0957-4484/26/45/455401 |
| [31] |
Z. Xing, Z. Ju, J. Yang, H. Xu, Y. Qian, Nano Res. 5 (2012) 477-485. DOI:10.1007/s12274-012-0233-2 |
| [32] |
A. Jayakumar, R.P. Antony, R. Wang, J. Lee, Small 13 (2017) 1603102. DOI:10.1002/smll.201603102 |
| [33] |
M. Ghimire, S. Bhoyate, R.K. Gupta, et al., J. Nanosci. Nanotechnol. 19 (2019) 4481-4494. DOI:10.1166/jnn.2019.16644 |
| [34] |
F. Zheng, D. Zhu, Q. Chen, ACS Appl. Mater. Interfaces 6 (2014) 9256-9264. DOI:10.1021/am501512j |
| [35] |
(a) S. Chen, M. Xue, Y. Li, et al., Inorg. Chem. Front. 2(2015) 177-183; (b) F. Wang, Y. Liu, Y. Zhao, et al., Appl. Sci. Basel 8(2018) 22. |
| [36] |
H. Che, A. Liu, J. Mu, et al., Electrochim. Acta 225 (2017) 283-291. DOI:10.1016/j.electacta.2016.12.164 |
| [37] |
S. Chen, M. Xue, Y. Li, et al., J. Mater. Chem. A Mater. Energy Sustain. 3 (2015) 20145-20152. DOI:10.1039/C5TA02557E |
| [38] |
R.R. Salunkhe, Y.V. Kaneti, Y. Yamauchi, ACS Nano 11 (2017) 5293-5308. DOI:10.1021/acsnano.7b02796 |
| [39] |
Y. Lei, J. Li, Y. Wang, et al., ACS Appl. Mater. Interfaces 6 (2014) 1773-1780. DOI:10.1021/am404765y |
| [40] |
H. Jiang, J. Ma, C. Li, Chem. Commun. (Camb.) 48 (2012) 4465. DOI:10.1039/c2cc31418e |
| [41] |
F.X. Ma, L. Yu, C. Xu, X.W.D. Lou, Energy Environ. Sci. 9 (2016) 862-866. DOI:10.1039/C5EE03772G |
| [42] |
R.P. Antony, A.K. Satpati, K. Bhattacharyya, B.N. Jagatap, Adv. Mater. Interfaces 3 (2016) 1600632. DOI:10.1002/admi.201600632 |
| [43] |
(a) L. Wu, Z. Wang, Y. Long, et al., Small 13(2017) 1604270; (b) S. Zheng, X. Guo, H. Xue, et al., Chem. Commun. 55(2019) 10904-10907. |
| [44] |
Y. Li, P. Hasin, Y. Wu, Adv. Mater. 22 (2010) 1926-1929. DOI:10.1002/adma.200903896 |
| [45] |
T.N. Lambert, J.A. Vigil, S.E. White, et al., Chem. Commun. 51 (2015) 9511-9514. DOI:10.1039/C5CC02262B |
| [46] |
Z. Wu, A. Winter, L. Chen, et al., Adv. Mater. 24 (2012) 5130-5135. DOI:10.1002/adma.201201948 |
| [47] |
D. Jiang, M. Zheng, Y. You, et al., RSC Adv. 8 (2018) 31853-31859. DOI:10.1039/C8RA04827D |
| [48] |
B. Xue, K. Li, S. Gu, J. Lu, J. Colloid Interface Sci. 530 (2018) 233-242. DOI:10.1016/j.jcis.2018.06.077 |
 2020, Vol. 31
2020, Vol. 31 

