b State Key Lab of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai 200050, China
Hydrogen sulfide (H2S) is a sort of toxic, corrosive and inflammable gas. H2S gas is normally generated from sewage treatment plants, manure-handling plants, tanneries and petroleum refineries, and it is widely utilized in various chemical industries. When the gas concentration of H2S is higher than 250 ppm, it may lead to the death of human beings [1]. Even at lower concentration levels, H2S is harmful to human health. H2S poisoning is the second most common cause of death in workplaces, after carbon monoxide poisoning [2]. On the other hand, H2S is found to be a kind of endogenous gases. It plays a role in cellular signaling, and represents the third gasotransmitter after nitric oxide and carbon monoxide. It can be found in the exhaled breath of human beings, and is used as breath marker for halitosis [3-5]. It appears to be elevated in asthma, and could be a biomarker of neutrophilic-type asthma [6, 7]. Such H2S detection at sub-ppm or lower concentrations is highly demanded. In most cases, GC–MS (gas chromatography-mass spectrometer) is used for the precise detection of traces H2S. However, GC–MS is sensitive but bulky and costly. Chemical sensors based on metal-oxide semiconductor sensing [8], electrochemical detection [9] and optical measurement [10] are developed. However, these sensors are portable but with low sensitivity or selectivity. H2S sensors that are sensitive, cheap, portable and selective are still research hotspots.
Herein we construct a novel H2S sensor based on integrated resonant dual-microcantilevers. Resonant microcantilever sensors, which can transduce adsorbed mass into resonant frequency shift, have been proven highly potential in trace-level gas sensing applications [11-17]. In this study, two microcantilever sensors with the same geometrical dimensions but different sensing materials are combined to identify and detect trace-level H2S gas. The schematic view of the combined sensor structure and the working mechanism is illustrated in Fig. 1. One microcantilever sensor (denoted as sensing cantilever) is loaded with zeolitic imidazolate framework (ZIF-8) derived nitrogendoped nanoporous carbon (NPC). The NPC sensing material is with ultra-high specific surface area and extremely sensitive to H2S. However, it possesses the drawback of limited selectivity. The other cantilever sensor (denoted as identifying cantilever) is loaded with basic copper carbonate (BCC). BCC can react with H2S and lose weight, as indicated by the chemical reaction equation: Cu2(OH)2CO3 + 2H2S → 2CuS+3H2O↑ + CO2↑. Therefore, contrary to the sensing cantilever, the identifying cantilever will output positive frequency shift signals due to weight loss when immersed into H2S atmosphere. This unique property of BCC enables the identifying cantilever to identify H2S gas from a variety of common gases, for most other gases which can react or be adsorbed with BCC will make it to add weight. Unfortunately, the reaction between BCC and H2S is irreversible, therefore the identifying cantilever alone is not suitable for H2S detection. However, combined detection with the both cantilever sensors will benefit from their advantages. The identifying cantilever can identify whether the analyte gas contains H2S, while the sensing cantilever can measure the concentration of H2S gas. This new combined gas sensor is promising to be widely used for on-site rapid identification and detection to H2S.

|
Download:
|
| Fig. 1. Schematic view to illustrate the combined detection to H2S with the integrated resonant dual-microcantilevers combined sensor. | |
The micro-openings inter-etch & sealing (MIS) micromachining technology, which is IC-foundry compatible, low-cost and highyield, has been developed in our lab [18]. Based on this micromachining technology, a novel process steps are designed to fabricate the dual-microcantilevers structure. Fig. 2 shows the main fabrication process steps: (a) After thermal oxidation for the (111) wafer, piezo-resistors are formed by ion implantation. Then the oxidation layer is patterned by reactive-ion-etching (RIE). After that, silicon deep RIE (DRIE) is utilized to form the trenches. The trenches define the outline of the dual-microcantilevers. (b) Tetraethoxy-silane (TEOS) SiO2 layer is coated on the top and lateral surfaces of the trenches by low pressure chemical vapor deposition (LPCVD). After RIE for removal of the SiO2 layer on the top surface of the trenches, the trenches are deepened by DRIE. (c) The wafer is etched in tetramethylammonium hydroxide (TMAH) solution with a concentration of 25% at 80 C. According to the anisotropic etching rules for (111) silicon wafer, the etching occurs along the lateral direction to form a hollowed channel. (d) All the SiO2 layers are removed by buffered oxide etchant (BOE) wet etching. Then thermal oxidation is performed again to protect all the surfaces of the structure. (e) LPCVD poly-silicon is deposited to seal the narrow trenches, followed by a RIE process to remove the poly-silicon outside the sealing regions. (f) Then the interconnection contact holes are opened by RIE process, and Au/Cr thin-film is sputtered, patterned and wet etched to form metal interconnection lines. (g) The wafer is etched in 25% aqueous TMAH again to remove all the poly-silicon and free the dual-cantilevers structure. (h) To reduce the damping while the cantilever is vibrating, the silicon DRIE is used again to widen the gap between the edge of the cantilever and the substrate.

|
Download:
|
| Fig. 2. Fabrication processes of the integrated resonant dual-microcantilevers. | |
After the fabrication processes, the dual-microcantilevers structure is ready for sensing materials loading.
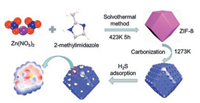
The BCC sensing material is bought from Sigma-Aldrich, while the NPC sensing material is synthesized in our lab. The synthesis processes of NPC are illustrated in Fig. 3. Firstly, the MOF (metal organic framework) material of ZIF-8 is synthesized using a solvothermal method. Then a ceramic boat with 0.6 g of dried ZIF-8 is placed in a flow-through tube furnace. The furnace is heated to 900 ℃ with the ramp of 10 ℃/min under nitrogen atmosphere, and then maintained at 900 ℃ for 5 h in a nitrogen atmosphere, followed by cooling down to room temperature. After that, the carbonized NPC powder was washed with 1 mol/L hydrochloric fluoride (HF) three times at room temperature to remove inorganic compounds, followed by a further wash with deionized water, then dried at 60 ℃ overnight.
|
Download:
|
| Fig. 3. The synthesis processes of NPC and the specific capturing mechanism for H2S molecules. | |
The synthesized NPC sensing material is characterized by TEM (FEI Tecnai G20). As is shown in Figs. 4a and b, the TEM images clearly show the nanoporous structure of the NPC, which indicates a large specific surface area of the sensing material. The elemental mapping images of EDS in Figs. 4c and d indicate that the NPC material contains a high density of basic-nitrogen sites which will enhance H2S adsorption capability.

|
Download:
|
| Fig. 4. (a, b) TEM and (c, d) EDS characterization results of the synthesized NPC material. | |
After that, the two kinds of sensing materials are loaded onto the dual-microcantilevers respectively through inkjet printing technology. Firstly, each 10 mg of the BCC and NPC sensing material is added into 1 mL of deionized water under ultrasonic to form crude suspensions as inks, respectively. Then, several drops of the two kinds of inks are printed onto the dual-microcantilever top surface respectively by using a commercial inkjet printer (GIX II Microplotter, Sonoplot Inc.). Thereafter, the dual-microcantilever sensor is dried in an oven at 60 ℃ for about 2 h.
The SEM images of the sensor chip and the dual-microcantilevers loaded with BCC and NPC sensing materials respectively are shown in Fig. 5. The sensor chip is extremely small and the dimension is as tiny as 0.7 mm × 0.7 mm × 0.45 mm.

|
Download:
|
| Fig. 5. (a) SEM micrographs of the tiny sensor chip and the (b) integrated resonant dual-microcantilevers with BCC and NPC sensing materials loaded respectively. | |
To verify the sensing mechanism of BCC sensing material to H2S gas, GC–MS is used to examine the reaction product between BCC and H2S, and the GC–MS spectrum is shown in Fig. 6. The existence of CO2 molecules verifies that BCC reacts with H2S gas and lose weight, which further confirms the working mechanism of the Identifying Cantilever.

|
Download:
|
| Fig. 6. GC–MS spectrum of the reaction product between BCC and H2S, which verifies the existence of CO2 molecules. | |
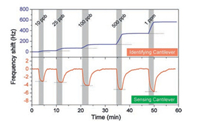
Then the sensor is put into a testing chamber with a dynamic volumetric method for preparing the standard gases for H2S detecting experiments. The gases which flow into the testing chamber are switched between H2S gas with different concentration and pure nitrogen in a constant flow rate by using a four-way valve. Fig. 7 shows the responses of the combined sensor to various concentrations of H2S ranging from 10 ppb to 500 ppb. As is predicted, the sensing responses of the Identifying Cantilever and the Sensing Cantilever are opposite. Meanwhile, the reaction between BCC and H2S is irreversible indeed, and the reaction will be continuous in the H2S atmosphere. As for the Sensing Cantilever, the adsorption of H2S onto NPC sensing material is reversible and saturable.
|
Download:
|
| Fig. 7. Responses of the resonant dual-microcantilevers combined sensor to various concentrations of H2S ranging from 10 ppb to 1 ppm. The responses of the two cantilevers are recorded simultaneously. | |
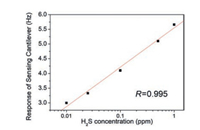
The absorption of H2S molecules onto NPC surfaces follows the Langmuir adsorption isotherm. As is shown in Fig. 8, the frequency-shift response of the Sensing Cantilever has a linear relationship with the H2S concentrations in a semilogarithmic coordinate system. Considering that the signal baseline is as low as ±0.1 Hz, the limit of detection (LOD) of the sensor to H2S can be calculated as below 1 ppb.
|
Download:
|
| Fig. 8. The relationship between the response of the sensing cantilever and the H2S concentrations. | |
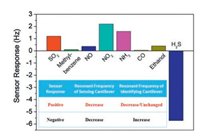
To investigate the identification capability of the dualcantilever sensor, seven kinds of common gases are chosen as the interfering gases. Fig. 9 shows the experimentally obtained results of the sensor to these interfering gases and H2S with the identical concentration of 1 ppm, respectively. Positive or negative sensor response is determined by the Identifying Cantilever, while the numerical value of the sensor response comes from the response of the Sensing Cantilever. Noting that the sensor only exhibits a negative sensor response to H2S gas, it can be concluded that the sensor can identify and detect tracelevel H2S gas.
|
Download:
|
| Fig. 9. Responses of the resonant dual-microcantilevers combined sensor to various interfering gases and H2S with the identical concentration of 1 ppm. | |
In summary, a novel integrated resonant dual-microcantilevers combined gas sensor which can identify and detect tracelevel H2S has been proposed. With NPC and BBC sensing materials loaded respectively, the sensor can distinguish H2S from a variety of common gases. The LOD of the combined sensor reaches as sensitive as below 1 ppb. Such an ultrasensitive and selective sensor is promising in H2S detection and monitoring applications.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research is supported by National Key R&D Program of China (No. 2016YFA0200800), National Natural Science Foundation of China (NSFC, Nos. 61874130, 61604163, 61527818, 61604162) and Key Research Program of Frontier Sciences of Chinese Academy of Sciences (No. QYZDJ-SSW-JSC001). Haitao Yu also appreciates the financial supportof the Youth Innovation Promotion AssociationCAS (No. 2017278).
| [1] |
M.D. Shirsat, M.A. Bangar, M.A. Deshusses, N.V. Myung, A. Mulchandani, Appl. Phys. Lett. 94 (2009) 083502. DOI:10.1063/1.3070237 |
| [2] |
J.F.D.S. Petruci, A.A. Cardoso, Anal. Chem. 88 (2016) 11714-11719. DOI:10.1021/acs.analchem.6b03325 |
| [3] |
S.J. Choi, B.H. Jang, S.J. Lee, et al., ACS Appl. Mater. Interfaces 6 (2014) 2588-2597. DOI:10.1021/am405088q |
| [4] |
M.F.R. Alomar, T.Q. Ruiz, D. Kurouski, et al., J. Phys. Chem. B 119 (2015) 1265-1274. DOI:10.1021/jp508471v |
| [5] |
Y. Zhou, W. Chen, J. Zhu, et al., Small 10 (2014) 4802. DOI:10.1002/smll.201470146 |
| [6] |
K.F. Chung, Expert Rev. Respir. Med. 8 (2014) 5-13. DOI:10.1586/17476348.2014.856267 |
| [7] |
M. Whiteman, P.K. Moore, J. Cell. Mol. Med. 13 (2009) 488-507. DOI:10.1111/j.1582-4934.2009.00645.x |
| [8] |
Y. Ren, X.Y. Yang, X.R. Zhou, et al., Chin. Chem. Lett. 30 (2019) 2003-2008. DOI:10.1016/j.cclet.2019.01.019 |
| [9] |
X. Liang, Y. He, F. Liu, et al., Sens. Actuator. B-Chem. 125 (2007) 544-549. DOI:10.1016/j.snb.2007.02.050 |
| [10] |
H.R. Zheng, L.Y. Niu, Y.Z. Chen, et al., Chin. Chem. Lett. 27 (2016) 1793-1796. DOI:10.1016/j.cclet.2016.04.023 |
| [11] |
H.T. Yu, P.C. Xu, X.Y. Xia, D.W. Lee, X.X. Li, IEEE Trans. Ind. Electron. 59 (2012) 4881-4887. DOI:10.1109/TIE.2011.2173094 |
| [12] |
H.T. Yu, X.X. Li, X.H. Gan, et al., J. Micromech. Microeng. 19 (2009) 045023. DOI:10.1088/0960-1317/19/4/045023 |
| [13] |
H.T. Yu, T.T. Yang, Y. Chen, et al., Anal. Chem. 84 (2012) 6679-6685. DOI:10.1021/ac3011022 |
| [14] |
H.T. Yu, P.C. Xu, D.W. Lee, X.X. Li, J. Mater. Chem. A 1 (2013) 4444-4450. DOI:10.1039/c3ta01401k |
| [15] |
Y.Q. Lv, P.C. Xu, H.T. Yu, J.Q. Xu, X.X. Li, Sens. Actuator. B. -Chem. 262 (2018) 562-569. DOI:10.1016/j.snb.2018.02.058 |
| [16] |
P.C. Xu, H.T. Yu, X.X. Li, Anal. Chem. 83 (2011) 3448-3454. DOI:10.1021/ac200015c |
| [17] |
Y.Y. Bao, P.C. Xu, S.R. Cai, H.T. Yu, X.X. Li, Talanta 182 (2018) 148-155. DOI:10.1016/j.talanta.2018.01.086 |
| [18] |
J.C. Wang, X.X. Li, IEEE Electron Dev. Lett. 32 (2011) 979-981. DOI:10.1109/LED.2011.2147272 |
 2020, Vol. 31
2020, Vol. 31 

