The flexible, sensitive, and portable chemical sensor to quick detect toxic gases, has gained special attention due to needs for environmental protection and healthcare as well as security [1]. Among diverse gas sensors, electrospun nanofibrous metal oxides (MOS) based gas sensors are of current interest for their large surface area and high aspect ratio, benefiting for gas adsorption and electrical signals transfer between two adjacent electrodes, thus high sensitivity and efficiency can be obtained [2]. Till now, a series of nanofibrous MOS based gas sensors, such as TiO2, ZnO, SnO2 and In2O3 have been employed in flexible sensors [3].
Trimethylamine (TMA, (CH3)3N) is a colorless, fishlike smell and toxic gas, which is usually produced during the deterioration process of seafood. Its amount can increase with the decomposition of trimethyl-N-oxide in seafood after its death, thus this gas can be chosen as an effective indicator of seafood quality [4]. To effectively detect TMA, several techniques, such as PH test, liquid chromatography, ion mobility spectrometry and mass spectrometry, have been employed [5], but flexible and portable TMA chemical sensors are still a challenge unsolved.
In this paper, we demonstrate a flexible TMA gas sensor based on In2O3 nanofibers. High response value, short response and recovery times, good reliability and flexibility are obtained. We believe our method can offer a powerful platform to understand and design high performance TMA sensors.
All chemicals (analytical grade reagents) were purchased from Beijing Chemicals Co., Ltd. and used as received without further purification. In2O3 nanofibers were prepared by an electrospinning method [6]. Typically, 0.42 g of In(NO3)3·4.5H2O was added to 8.8 g of mixed solvent contained N, N-dimethylformamide (DMF)/ethanol with the weight ratio of 1:1 and stirred for 2 h, and then 1.2 g of polyvinyl pyrrolidone (PVP) was added to the above solution with stirring for another 6 h. Electrospinning of In2O3 nanofibers was conducted with a similar set-up which has been detailed in a previous study [7]. The as obtained spinning solution was delivered to a hypodermic syringe at a constant flow rate of 1.0 mL/h, and then electrospun by applying 20 kV at an electrode distance of 25 cm. A piece of aluminum foil was used as the cathode and several sensor substrates were placed on it.
X-Ray diffraction (XRD) analysis was conducted on a Rigaku D/max-2500 X-ray diffractometer with Cu Kα radiation (λ =1.5418 Å). Field-emission scanning electron microscopy (FE-SEM) images were performed on a JEOL JEM-6700 F microscope operating at 5 kV. Transmission electron microscopy (TEM) images were obtained on a JEOL JEM-2000EX microscope with an accelerating voltage of 200 kV.
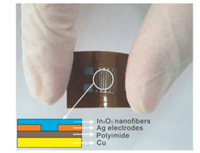
Polyimide/Cu substrates were pretreated by O2 plasma for ten minutes to increase its hydrophilicity. Ag nano ink was purchased from Daicel Co., Ltd. Ag microelectrodes were printed on the polyimide side of the polyimide/Cu substrates with a metal-jetting system (MJ-10, Elite Tech Co., Ltd., China) using a 10 pL cartridge. A customized waveform was set to match the inks with a drop velocity of approximately 5 m/s, a jetting voltage of 20 V, a cartridge print height of 0.6 mm, and a jetting frequency of 5.0 kHz. The cartridge and plate temperature was maintained at room temperature (25 ℃). Fine uniform Ag electrodes were formed with a width of 50 μm, a distance of 100 μm, and a thickness of 10 μm, followed by sintering at 80 ℃ for 30 min under ambient conditions to become highly conductive (Fig. 1).
|
Download:
|
| Fig. 1. The structure and a photograph of the flexible sensor. | |
The In2O3 nanofibers were spin-coated on polyimide/Cu substrates with Ag microelectrodes. In a typical procedure, the as-prepared nanofibers were mixed with deionized water (with a resistivity of 18.0 MV/cm) in a weight ratio of 100:5 to form a paste. The substrates were pretreated by O2 plasma for 10 min, and then dipped into the nanofiber-solution and spin-coated at 3500 rpm for 30 s. After that, the substrate was dried at 80 ℃ for 2 hbefore thenext step. This process was repeated for 4 times.
The synchronous analysis of strain, stress, electrical properties or gas sensing is provided by an AES-4SD Flexible Device Analysis System (Beijing Sinoagg Tech Co., Ltd., China). A force sensor in G1 level with the range of 5 N is used to accuracy control and feedback the strain of stretching, bending or pressing in real time. A fixture with conductive material is designed for connecting the flexible device and SA7102 Double Source Measure Unit (SMU, Beijing Sinoagg Tech Co., Ltd., China). A 300 mL gas chamber of AES-4SD is provided by closing the cover. All the test programs were controlled by the SA7102 software, especially for long-time fatigue testing of 100 cycles of stretching and electrical testing and photo or video recording in real time. The temperature and humidity were controlled at 80 ℃ and 25% RH, respectively. The sensitivity value (S) was defined as S = Ra/Rg, where Ra was the sensor resistance in air and Rg was a mixture of target gas and air. The time taken by the sensor to achieve 90% of the total resistance change was defined as the response time in the case of adsorption or the recovery time in the case of desorption. The schematic illustration of the experimental equipment is shown in Fig. 2.

|
Download:
|
| Fig. 2. A photograph of a flexible gas sensor in the analysis system. | |
Fig. S1 (Supporting information) shows the XRD pattern of In2O3 nanofibers. All the diffraction peaks coincide with the corresponding peaks of the cubic structure of In2O3 given in the standard data file (JCPD No. 89-4595). No diffraction peaks from any other impurities are detected.
Fig. S2 (Supporting information) displays a typical FE-SEM image of In2O3 nanofibers, indicating the product is highly dominated by the nanofibers with diameters ranging from 80 nm to 120 nm and lengths of several tens of micrometers. Further morphology characterization was performed on a transmission electron microscope (TEM) and shown in the insert. The nanofibers are consisting of nanoparticles with an average diameter of about 30 nm. The coarse and loose grains within nanofibers can adsorb more gas molecules on the fiber surface, which improve the sensing properties eventually.
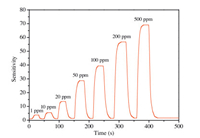
The sensor was exposed to different concentrations of TMA gases at 80 ℃. Fig. 3 shows the dynamic response-recovery curve of the flexible sensor with different TMA concentrations. The sensor has a wide response range for TMA of 1-500 ppm. When exposed to 1 ppm of TMA, the sensitivity (Ra/Rg) is about 3.8. The response increases significantly with increasing gas concentration. The sensitivities are about 5.5, 13.6, 28.7, 39.4, 56.4 and 69.1–10, 20, 50, 100, 200 and 500 ppm TMA gas, respectively. In addition, the flexible sensor also exhibits quick response and recovery toward TMA gas, and the average response and recovery times are measured to be 6 and 10 s, respectively.
|
Download:
|
| Fig. 3. Response and recovery curves of the sensor to different concentrations of TMA gases. | |
The response and recovery curves of the sensor to 500 ppm TMA gas were collected at 25%, 45% and 80% RH conditions (Fig. S3 in Supporting information). The sensitivity decreases evidently with increasing RH. The sensitivity is about 54 when the sensor was exposed to 500 ppm TMA gas at 45% RH, and the value is only about 15.3 at 80% RH. Similar results are reported in many other papers [6], and because In2O3 is also a humidity-sensing material, the current sensor can only work in dry environments.
The sensitivities of sensor to 500 ppm different gases are shown in Fig. S4 (Supporting information). The sensor shows less sensitive to C2H5OH, CH3OH, CH3COCH3 and C2H2 than to TMA gas, and totally insensitive to H2, CH4, CO and NOx. In2O3 is widely reported to response to both oxidizing and reductive gases [3], thus undoped In2O3 nanofibers can react with many gases.
The flexibility of the sensor was investigated by 100 bending/extending cycles to 100 ppm TMA gas. The sensitivities are recorded and shown in Fig. 4a. No obvious changes for the sensing performance can be obtained after 100 bending/extending cycles, revealing the reliable and excellent flexibility of the sensors. Moreover, bending tests of the fabricated sensors were carried out at various curvature angles (0°, 30°, 60° and 90°) to 100 ppm TMA gas (Fig. 4b). As the bending increases, the strain between the substrate and the sensing layer results in quite a minor change in the surface resistance, indicating an excellent flexibility of the obtained sensor.

|
Download:
|
| Fig. 4. Sensitivities of the sensors to 100 ppm TMA gas (a) after different bending cycles and (b) at different curvature angles. | |
In2O3 is a topical gas sensing material due to its abilities such as adsorption and reaction of gas molecules [8]. Oxygen molecules, which are chemisorbed on the nanofibers, can generate oxygen species and make the nanofibers less conductive. When the sensor was exposed to TMA, TMA molecules may react with the chemisorbed oxygen species and release the trapped electron back to the conduction band, and the sensor resistance is decreased accordingly. This trend can be represented by the following equation [9]:
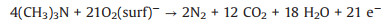
|
The excellent sensing performances and good flexibility are also related to the one-dimension nanostructure of the nanofibers, which owns high surface energies, varied nanofiber-to-nanofiber junctions, and large surface-to-volume ratios [10]. These characteristics lead both high flexibilities and effective electron transports, and show high sensitivity, quick response-recovery, and good mechanical restorability.
In conclusion, a novel flexible TMA gas sensor has been demonstrated. High response value, short response and recovery times, good reliability and flexibility are observed at 80 ℃. The sensitivity is up to 3.8 when the sensor was exposed to 1 ppm TMA gas, and the average response and recovery times are 6 and 10 s, respectively. These results demonstrate that our sensor is a potential candidate as high-performance TMA gas sensors.
Declaration of competing interestWe confirm that our work have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51808328 and 61903235), Natural Science Foundation of Shandong Province (Nos. ZR2017LEM010 and ZR2019BEM036).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.048.
| [1] |
B. Park, J. Kim, D. kang, C. Jeong, K.S. Kim, et al., Adv. Mater. 28 (2016) 8130-8137. DOI:10.1002/adma.201602425 |
| [2] |
F. Xu, Y. Zhu, Adv. Mater. 24 (2012) 5117-5122. DOI:10.1002/adma.201201886 |
| [3] |
Y. Ma, Y. Zhang, S. Cai, Z. Han, X. Liu, et al., Adv. Mater. (2019) 1902062. |
| [4] |
Y. Cui, Q. Wei, H. Par, C.M. Lieber, Science 293 (2001) 1289-1292. DOI:10.1126/science.1062711 |
| [5] |
Z.W. Pan, Z.R. Dai, Z.L. Wang, Science 291 (2001) 1947-1949. DOI:10.1126/science.1058120 |
| [6] |
Q. Qi, T. Zhang, L. Wang, Appl. Phys. Lett. 93 (2008) 023105. DOI:10.1063/1.2957477 |
| [7] |
Y. Zhang, X. He, J. Li, Z. Miao, F. Huang, Sens. Actuator. B-Chem. 132 (2008) 67-73. DOI:10.1016/j.snb.2008.01.006 |
| [8] |
A. Kolmakov, M. Moskovits, Ann. Rev. Mater. Res. 34 (2014) 151-180. |
| [9] |
Y.C. Lee, H. Huang, O.K. Tan, M.S. Tse, Sens. Actuator. B-Chem. 1321 (2008) 239-242. |
| [10] |
P.G. Su, C.L. Uen, Talanta 66 (2005) 1247-1253. DOI:10.1016/j.talanta.2005.01.039 |
 2020, Vol. 31
2020, Vol. 31 

