b Department of Physics, College of Science, Shanghai University, Shanghai 200444, China
Amphetamines are one of the eight major drugs. The main component of drugs is organic amines which would cause air pollution and pose a threat to human health. Because organic amines are widely used in industrial production and pharmaceutical manufacturing [1], conveniently detection of organic amines has attracted more and more attention. Conventional amine- containing drug tests include blood test, sweat test, hair test, and saliva test, while many inadequacies are reflected in the fact that the inspection equipment is expensive, limited and susceptible to contamination by other substances. Over the years, analytical chemistry has been applied to the detection of organic amines, such as thin layer chromatography (TCL), GC-MS, high performance liquid chromatography (HPLC) and other immunoassay [2].
Quartz crystal microbalance (QCM) is a kind of gas sensor with many advantages such as high sensitivity [3-6], convenient production, low price, easy digitization, wide application [7-9] and long distance measurement [10-13]. At present, QCM have been used in the detection of organic amines. For example, GO/Cu2O nanocomposite based QCM gas sensor for trimethylamine detection under low concentrations [14], QCM sensor for detection of aliphatic amines vapours [15], GO/chitosan nanocomposite coated QCM sensor for detection of amine vapors [16], slective chiral recognition of alanine enantiomers by chiral calix[4]arene coated QCM sensors [17]. However, these reports are rarely specific for the detection of amine-containing drugs. Some disadvantages generate from complicated and cumbersome material synthesis [18], and relatively expensive detection instrument.
Among the organic amines, β-phenylethylamine is a trace, endogenous amine, an isomer of Philopon, and a reagent that can excite human nerves. Chemical synthesis can also be achieved by illegal molecules. Therefore, the preparation of a sensitive, stable phenethylamine gas sensor is very helpful for the detection of amine-containing drugs. The calixarene is a kind of oligomeric cyclic compound, the lower edge is a neatly arranged hydrophilic phenolic hydroxyl group, and the size of the hydrophobic cavity formed by the benzene ring can be freely adjusted according to different reaction conditions [19, 20]. The calixarene is easily modified by functional groups forming a complex with a substrate and a neutral molecule [21, 22]. So this advantage can be used to make a new underlay for the synthesis of novel calix-[6]arene derivativesis. It has high melting point, good thermal stability and chemical stability, as well as low toxicity and flexibility. In order to increase the surface area, further improve its performance, we synthesized a novel composite material with GO [23-25].
In this work, a novel calix[6]arene (p-acetylcalix[6]arene) is synthesized through the methods of acylation and esterification and ketocarbonyl is introduced into the structure. Then GO is used to composite with this p-acetylcalix[6]arene and get the composite of both (see Section 1 in Supporting information for details). The composite we obtained has a high selective recognition of β-phenylethylamine. Bycomparisonwiththose methods in literature above, our method has high selectivity and sensitivity to amine- containing drugs, and the synthesis of raw materials is relatively simple. It is an innovative breakthrough in QCM.
The FT-IR spectra was used to characterize p-acetylcalix[6]-arene, GO and the composite of both (Fig. S1 in Supporting information). Both the peaks of GO and p-acetylcalix[6]arene appears in the complex includes, so the composite of both was successfully doped. Raman spectrum can also prove the successful composition of the material (Fig. S2 in Supporting information). The characteristic peaks of GO are weakened and the intensity of the absorption peak for composite is enhanced.
The surface topography of the sample was microscopically imaged by scanning electronic microscopy (SEM). From the Fig. 1, it can be observed that the p-cetylcalix[6]arene is a regular rod-like structure [26]. Fig. 2 presented the internal crystal structure of the sample are characterized Transmission Electron Microscope (TEM).

|
Download:
|
| Fig. 1. The SEM images of p-acetylcalix[6]arene. (a) Scale bar=400 nm; (b) Scale bar = 50 nm. | |

|
Download:
|
| Fig. 2. (a, b) The TEM image of p-acetylcalix[6]arene at different scales. | |
The fluorescence spectrum of the reaction of p-acetylcalix[6]arene with β-phenethylamine is shown in the Fig. 3 [27]. It can be known that the excitation and emission spectra of the new calixarene derivative without reaction with β-phenethylamine liquid are 325 nm and 385 nm, respectively, and the strength is high. After reacting with β-phenethylamine liquid, both the excitation and emission spectra are red shifted. The absorption peak appears at 375 nm and 450 nm, and the intensity decreases. Fluorescence analysis just coincides with a schiff base mechanism, the amino group is a color-assisting group. During the reaction between the carbonyl group and amino group, a large number of color-promoting groups are introduced, so that the peak is red- shifted [28]. The Schiff base reaction produces a large amount of ionic intermediates in a reversible reaction, resulting in destruction of its conjugated structure, so that the fluorescence intensity is lowered.

|
Download:
|
| Fig. 3. The fluorescence spectrum of the reaction of p-acetylcalix[6]arene with β-phenethylamine. | |
In Fig. 4, it can be analyzed by SEM image that when the mass ratio of GO to p-acetylcalix[6]arene is 1:4.5, the rod-shaped p-acetylcalix[6]arene was observed to be uniformly and nonstacked on the sheet-like GO. This composite of both not only makes full use of multiple carbonyl groups on the novel p-acetylcalix[6]arene, but also utilizes the large specific surface area of the sheet of GO, combining the advantages of both to achieve high selectivity and adsorption of amine-containing drugs.

|
Download:
|
| Fig. 4. The SEM images of different mass ratios of GO and p-acetylcalix[6]arene. (a) GO:p-acetylcalix[6]arene = 1:4; (b)GO:p-acetylcalix[6]arene = 1:4.5; (c) GO:p-acetylcalix-[6]arene= 1:5. | |
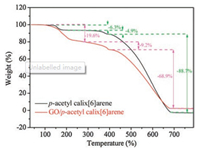
For the study of material components, thermogravimetric analysis is a commonly used analytical method. As shown in Fig. 5, the first weight loss peak temperature of the p-acetylcalix[6]arene is between 145 ℃ and 180 ℃, and the weight loss rate is 6.3%, resulting from dehydration and weight loss ofhydroxyl groups. The second weight loss peak temperature is between 350-390 ℃, and the weight loss rate is 4.9%, which is caused by demethylation. The third weight loss peak can be found between 400-680 ℃ and the weight loss rate is 88.8%, which is the collapse of the calixarene ring structure. Due to the incorporation of GO, the weight loss rate in the temperature range of 200-700 ℃ is lower than that of the p-acetylcalix[6]arene [29].
|
Download:
|
| Fig. 5. Image of the thermogravimetric analysis diagram of 0-800 ℃ air blowing. | |
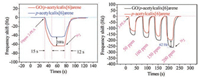
Fig. 6 shows the response of p-acetylcalix[6]arene and its complex to β-phenylethylamine adsorption at a low concentration of 10 ppm, with good response (15 s) and recovery time (12 s). And at 10 ppm phenylethylamine, the composite ofboth is 20 Hz higher than para-acetylcalixarene adsorption. Gradient tests show that as the concentration increases, the response of the compound gradually increases, and the gas response of the compound about 40 Hz higher than that of p-acetylcalix[6]arene. This advantage of the compound is even more pronounced at high concentrations. The principle ofthe QCM gas-sensitive elements, basic devices, and basic detection principles ofgas on QCM are introduced in detail in Section 3 in Supporting information.
|
Download:
|
| Fig. 6. The gradient test of 50 ppm, 100 ppm, 150 ppm, 200 ppm β-phenylethylamme vapor. | |
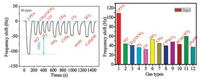
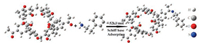
As we all know, the response time is the time required to contact a certain concentration of the measured gas to reach a stable frequency. Recovery time is the desorption time of the detected gas after N2 blowing. Fig. 6 shows that at 10 ppm phenylethylamine gas concentration, the response time is less than 15 s, and the recovery time is less than 12 s, so it has excellent response andrecovery dynamics. Compared with the adsorption and desorption properties at a low concentration of 10 ppm, the higher the concentration, the better the sensitivity, and the adsorption response time and the desorption response time are all lower than 10 s. In Fig. 7, we can see that at 40 ppm, the adsorption of β-phenethylamine has an advantage of about 50 Hz over other gases so p-acetylcalix[6]arene has excellent selectivity. At room temperature 25 ± 1 ℃, ambient humidity is 45%, using different saturated aqueous solutions ofLiCl, MgCl2, Mg(NO3)2, NaCl, KCl and KNO3 to achieve different gradient humidity control. The relative humidity was 11.3%, 33.3%, 55.87%, 75.61%, 85.92% and 95.41%, respectively, and humidity measurement experiments were performed within this range. Humidity and repeatability tests show that humidity has little effect on calixarene adsorption to amines and it has good stability in a month (Figs. S6 and S7 in Supporting information). In summary, the performance of such QCM sensors for the detection of amine-containing drugs is attributed to the following factors: The six hydroxyl groups introduced on the acetyl calixarene can undergo a Schiff base reaction with the amino group [30-32]. Mechanism reaction is shown in Fig. 8. The lone electron on β-phenylethylamine attacks the carbonyl carbon, and an addition elimination reaction occurs. The imine is unstable, so a reversible reaction occurs.
|
Download:
|
| Fig. 7. The selectivity of the 12 gases (QCM sensor coated with p-acetylcalix[6]arene). | |

|
Download:
|
| Fig. 8. Mechanism of Reaction of p-acetylcalix[6]arene with β-phenethylamine. | |
In order to verify the adsorption mechanism of p-acetylcalix[6] arene and β-phenylethylamine, we calculated the adsorption enthalpy and molecular configuration between molecules by Gaussian simulation (Table S1 in Supporting information). As shown in Fig. 9, the enthalpy change (ΔH) is -52.2 kJ/mol. According to the classical physicochemical adsorption theory, when the AH value is in the range of -80 kJ/mol to -40 kJ/mol, reversible chemisorption is very suitable for meeting the needs of gas sensors.
|
Download:
|
| Fig. 9. Adsorption enthalpy of Schiff base between p-acetylcalix[6]arene and amine groups of p-hydroxybenzaldehyde. | |
In this paper, organic synthesis is used to introduce a carbonyl group onto calix[6]arene and constructing QCM high frequencygas sensor. The amine-containing drugs are detected by the characteristics of their groups. The results show that the novel calix[6]arene derivative sensor not only shows good response to β-phenethyl-amine at low concentrations (10 ppm), but also exhibits excellent selectivity, sensitivity, long-term stability and repeatability. Through thermodynamic experiments and Gaussian simulations and fluorescence detection, consistent results can be obtained, namely the reversible schiffbase mechanism principle of carbonyl and amino groups. The composite of GO and p-acetylcalix[6]arene aromatic materials notonly increases the specific surface areaof the material, but also disperses the material into a sheet structure, further promoting the advantages of the sensor and the drug sensitivity detection field.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors acknowledge the support of National Natural Science Foundation of China (No. 61527818) and the Shanghai Municipal Education Commission (No. Peak Discipline Construction Program).
Appendix A. Supplementary dataSupplementary material related to thisarticle can be found, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2020.01.034.
| [1] |
Y. Lv, H. Yu, P. Xu, J. Xu, X. Li, Sensor. Actuat. B-Chem. 256 (2018) 639-647. DOI:10.1016/j.snb.2017.09.195 |
| [2] |
R. Toniolo, A. Pizzariello, N. Dossi, et al., Anal. Chem. 85 (2013) 7241-7247. DOI:10.1021/ac401151m |
| [3] |
Y. Zhu, Z. Cheng, Q. Xiang, Y. Zhu, J. Xu, Sensor. Actuat. B-Chem. 256 (2018) 888-895. DOI:10.1016/j.snb.2017.10.029 |
| [4] |
Y. Lu, S. Song, C. Hou, et al., Chin. Chem. Lett. 29 (2018) 65-68. DOI:10.1016/j.cclet.2017.08.003 |
| [5] |
H. Wang, X. Liu, J. Xie, M. Duan, J. Tang, Chin. Chem. Lett. 27 (2016) 464-466. DOI:10.1016/j.cclet.2015.12.027 |
| [6] |
L. Wang, Y. Yu, Q. Xiang, et al., Sensor. Actuat. B-Chem. 255 (2018) 2704-2712. DOI:10.1016/j.snb.2017.09.082 |
| [7] |
Z. Duan, Y. Jiang, M. Yan, et al., ACS Appl. Mater. Interfaces 11 (2019) 21840-21849. DOI:10.1021/acsami.9b05709 |
| [8] |
S. Wang, G. Xie, Y. Su, et al., Sensor. Actuat. B-Chem. 255 (2018) 2203-2210. DOI:10.1016/j.snb.2017.09.028 |
| [9] |
D. Battal, S. Akgonullu, M.S. Yalcin, et al., Biosens. Bioelectron. 111 (2018) 10-17. DOI:10.1016/j.bios.2018.03.055 |
| [10] |
L. Wang, Y. Zhu, Q. Xiang, et al., Sensor. Actuat. B-Chem. 251 (2017) 590-600. DOI:10.1016/j.snb.2017.05.074 |
| [11] |
L. Wang, X. Cha, Y. Wu, et al., ACS Omega 3 (2018) 2437-2443. DOI:10.1021/acsomega.8b00061 |
| [12] |
L. Wang, J. Gao, J. Xu, Sensor. Actuat. B-Chem. 293 (2019) 71-82. DOI:10.1016/j.snb.2019.04.050 |
| [13] |
L. Wang, Z. Wang, Q. Xiang, et al., Sensor. Actuat. B-Chem. 248 (2017) 820-828. DOI:10.1016/j.snb.2016.12.015 |
| [14] |
W. Chen, F. Deng, M. Xu, et al., Sensor. Actuat. B-Chem. 273 (2018) 498-504. DOI:10.1016/j.snb.2018.06.062 |
| [15] |
M.M. Ayad, N.L. Torad, Sensor. Actuat. B-Chem. 147 (2010) 481-487. DOI:10.1016/j.snb.2010.03.064 |
| [16] |
K. Zhang, R. Hu, G. Fan, et al., Sensor. Actuat. B-Chem. 243 (2017) 721-730. DOI:10.1016/j.snb.2016.12.063 |
| [17] |
F. Temel, S. Erdemir, B. Tabakci, et al., Anal. Bioanal. Chem. 411 (2019) 2675-2685. DOI:10.1007/s00216-019-01705-5 |
| [18] |
Y. Liu, Z. Zhong, J. Macromol. Sci. Part A 54 (2017) 678-683. DOI:10.1080/10601325.2017.1321959 |
| [19] |
D.S. Guo, Y. Liu, Chem. Soc. Rev. 41 (2012) 5907-5921. DOI:10.1039/c2cs35075k |
| [20] |
R.K. Castellano, D.M. Rudkevich, J. Julius Rebek, Nat. Acad. Sci. 94 (1997) 7132-7137. DOI:10.1073/pnas.94.14.7132 |
| [21] |
J. Rebek Jr, Chem. Commun. (2000) 637-643. DOI:10.1039/A910339M |
| [22] |
D.M.H.A.C. Redshaw, Chem. Rev. 108 (2008) 5086-5130. DOI:10.1021/cr8002196 |
| [23] |
D.R. Dreyer, S. Park, C.W. Bielawski, et al., Chem. Soc. Rev. 39 (2010) 228-240. DOI:10.1039/B917103G |
| [24] |
M. Hirata, T. Gotou, S. Horiuchi, et al., Carbon 42 (2004) 2929-2937. DOI:10.1016/j.carbon.2004.07.003 |
| [25] |
N.I. Zaaba, K.L. Foo, U. Hashim, et al., Proc. Engin. 184 (2017) 469-477. DOI:10.1016/j.proeng.2017.04.118 |
| [26] |
I.A. Koshets, Z.I. Kazantseva, Y.M. Shirshov, et al., Sensor. Actuat. B-Chem. 106 (2005) 177-181. DOI:10.1016/j.snb.2004.05.054 |
| [27] |
J.H. Wosnick, T.M. Swager, Chem. Commun. (2004) 2744-2745. DOI:10.1039/b411489b |
| [28] |
M. Namba, M. Sugawara, P. Buhlmann, et al., Am. Chem. Soc. 11 (1995) 635-638. DOI:10.1023/A:1021373400660 |
| [29] |
A. Ganguly, S. Sharma, P. Papakonstantinou, et al., J. Phys. Chem. C 115 (2011) 17009-17019. DOI:10.1021/jp203741y |
| [30] |
E. Valeur, M. Bradley, Chem. Soc. Rev. 38 (2009) 606-631. DOI:10.1039/B701677H |
| [31] |
C.L. Aronson, D. Beloskur, I.S. Frampton, et al., Polym. Bull. 52 (2004) 409-419. DOI:10.1007/s00289-004-0305-x |
| [32] |
S. Ren, Z. Wu, Q. Guo, B. Shen, Catal. Lett. 145 (2014) 712-714. |
 2020, Vol. 31
2020, Vol. 31 

