Ammonia (NH3) is widely used in the industry. However, it is also a highly toxic gas. Ammonia-contaminated not only causes serious harm to the ecological environment, and will threaten human health. Even small amount of NH3 is very harmful to the human body [1, 2]. Therefore, many researchers have been looking to develop a sensitive NH3 gas sensor to monitor it. Previous works have achieved great success in metal oxide-based gas sensors, due to the high sensitivity, good stability, low cost together with simple fabrication techniques [3-9]. Among them, indium trioxide (In2O3) is a traditional n-type semiconductor with a wide band gap that has been reported as a promising candidate for NH3 sensor. For example, B. Bessaïs et al. have reported tin-doped indium oxide (ITO) films prepared by the screen printing technique, which showed good sensing properties toward NH3 vapours [10]. However, the practical application of In2O3-based gas sensor is similar to most of the semiconductor type gas sensors, which is confined by their high working temperature. The sensor will not operate at low temperature without additional power supply, which critically limits their adaptation for practical deployment [11-13]. Therefore, the realization of high-performance roomtemperature NH3 sensors has been an urgent task.
Recently, two-dimensional (2D) materials such as graphene, transition-metal sulfides, and recently transition-metal carbides and their hybrids have shown promising properties for gassensing applications [14-17]. As a rising star of carbon family, reduced graphene oxide (rGO) with thick planar sheet comprising a sp2-bonded carbon structure, has attracted the attention of many scientists and researchers in various fields due to its intriguing properties of flexibility, good conductivity, superior chemical stability, large surface-to-volume ratio [18, 19]. In recent years, metal oxide composited with rGO to be a gas sensor attracted enormous interests [20-23]. For instance, Zeng et al. successfully prepared ternary NiO-SnO2-rGO nanocomposites. Gas-sensing results revealed that the ternary nanocomposites exhibited a remarkably higher response to NO2 at room temperature [24]. Inspired by the aforementioned concepts, the formation of a heterojunction by introducing rGO into In2O3 composites will enhance the NH3-sensing performance. Numerous studies have proved that the heterojunction formed by n-type metal oxide and p-type rGO can play a positive role in the sensing process. However, to the best of our knowledge, the rGO/In2O3 nanocomposites for the ammonia detection at room temperature are seldom mentioned.
Herein, to obtain a new sensingmaterial, we report the successful decoration of porous In2O3 nanocubes on the surface of rGO nanosheets via a facile hydrothermal method combined with subsequent annealing process.Themicrostructure andmorphology of the as-prepared nanocomposites were examined by various analytical methods. Experimental results indicated that through introduction of rGO, the sensing response of rGO/In2O3 nanocomposites towards NH3 is carried out at room temperature, which is expected to have a promising future for NH3 detection.
In this work, all the chemicals used were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd., China. The graphene oxide was prepared by the modified Hummer's method [25]. Porous In2O3 nanocubes were prepared using a hydrothermal reaction [26].100 mg of In2O3 nanocube was dissolved in 100 mL of ethanol and sonicated for 30 min. Then, 1 mL of APTES is added to the solution and stirred for 4 h at 60 ℃ to make the cube indium oxide surface with a positive charge. The solution was washed with ethanol and dried. This is in order to combine the two components with the negatively charged GO under electrostatic attraction. We dissolve 1 mg of GO in 80 mL of deionized water and disperse ultrasonically for 30 min, stir and add well-treated In2O3 to compound, after 30 min, transfer the mixture solution to 100 mL stainless steel autoclave, rise temperature to 120 ℃ and keep this temperature for 14 h. The product obtained was rGO/In2O3 nanocomposites and the product was washed with water and ethanol to dry. The fabrication and measurement of gas sensors are similar to our previous reported paper [27], which is shown in Fig. S1 (Supporting information).
Fig. 1a shows the XRD patterns of GO, rGO and rGO/In2O3 nanocomposites. As we can see from the GO pattern, the diffraction peak at around 2θ = 11.6° belongs to the (001) reflection of GO and no miscellaneous peaks were detected. The diffraction peaks at around 2θ = 24.5° and 43.4° belongs to the (002) and (100) reflection of rGO. These diffraction peaks indict that GO has been reduced to rGO after the hydrothermal treatment. Obviously, the XRD patterns of rGO/In2O3 nanocomposites are indexed to pure In2O3 (JCPDS No. 06-0416). Except for the diffraction peak of In2O3, no diffraction peak of graphene is observed in this XRD pattern. It can be attributed to the rather low amount of rGO sheets in the composites, as well as the relative high intensity of the In2O3. Fig. 1b shows Raman spectra of GO and rGO/In2O3 nanocomposites exhibited two major peaks ascribed to the D band at 1352.8 cm-1, the G band at 1596.5 cm-1, respectively, indicating the recovery of the pristine graphene structure. In addition, after the synthesis of rGO/In2O3 nanocomposites, the ID/IG ratio of nanocomposites is about 1.04, which confirms that the samples contain graphite carbon structure, so the conductivity of rGO/In2O3 nanocomposites will be improved. It is possible to increase gas-sensing properties.

|
Download:
|
| Fig. 1. (a) XRD patterns of GO, rGO and rGO/In2O3 nanocomposites. (b) Raman spectra of GO and rGO/In2O3 nanocomposites. | |
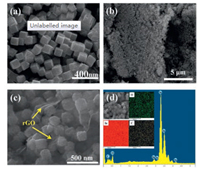
From the SEM image (Fig. 2a), it can be found that the morphology of the obtained In2O3 is cubic and these nanocubes are evenly dispersed with edge length of about 200 nm, and the size is relatively uniform. After composited with rGO, In2O3 nanocubes are dispersed uniformly the surface of the rGO (Figs. 2b and c). It contributes to alleviate the aggregation phenomenon, thereby improving the specific area of the samples. According to the EDS energy spectrum diagrams, the as-prepared samples are composed of three elements: C, O and In (Fig. 2d) and compared with O and In element, the amount of C element is less from the EDS mapping images.
|
Download:
|
| Fig. 2. (a) SEM image of In2O3 nanocubes. (b, c) SEM images and (d) EDS energy spectrum diagram of rGO/In2O3 nanocomposites. | |
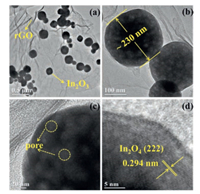
Moreover, the microstructure was also illustrated under transmission electron microscopy (TEM) to offer additional perception about the structure and morphology of the rGO/In2O3 nanocomposites. In religious accordance with the SEM consequences, a low magnification TEM photograph as is shown in Fig. 3a that a large amount of In2O3 nanocubes are regulating distribution on the rGO sheet. As is shown in Fig. 3b, it retained the original morphology of In2O3 nanocubes are still remained. In addition, Fig. 3c exhibits that the In2O3 nanocubes possess the porous structures, which can be further verified by the BrunauerEmmett-Teller (BET) characterization from Fig. S2 (Supporting information). According to figure, the N2 adsorption-desorption isotherm belongs to Type-Ⅳ isotherm according to IUPAC classification, which indicates there is a mesoporous structure in the sample. The specific surface area of rGO/In2O3 nanocomposites is about 46.3 m2/g and the pore size of samples focuses on 8.2 nm, which is extremely beneficial to the transmission and diffusion of gas molecules in the adsorption and desorption. Fig. 3d illustrates the HRTEM image of In2O3 nanocubes. A cluster of unique lattice fringes with a spacing of 0.294 nm consistent with the value of the (222) plane can be aboded in the HRTEM photograph of In2O3 nanospheres.
|
Download:
|
| Fig. 3. (a, b) Low magnification TEM images, (c) high magnification TEM image and (d) HRTEM image of rGO/In2O3 nanocomposites. | |
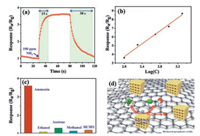
Fig. S3 (Supporting information) shows the cross-section of the alumina tube and full view of the film which is composed of rGO/In2O3 nanocomposites. It can be seen that most of the samples still remain in their initial sizes and connect to each other by physical attachment, forming a sensing film with the thickness of about 12.5 μm. In order to demonstrate the potential application in gas detection, the gas-sensing performances of rGO/In2O3 nanocomposites were investigated at room temperature. As shown in Fig. 4a, sensor response to 100 ppm NH3 is 3.5 at room temperature. The response and recovery times of gas sensors are very important to their practical applications, which are defined as the time for the sensor required to reach 90% of the maximal response upon exposure to the target gas, and the time of the sensor response decreasing to 10% of the stabilized value in the target gas after placing in clean air, respectively [28]. It can be found that the sensing performances of NH3 exhibited short response and recovery times, which are ~15 s and ~38 s, respectively. Fig. S4 (Supporting information) presents the recycling stability of sensors to 100 ppm NH3 at room temperature, indicating the good gas-sensing reproducibility. Concentration-dependent response curve of rGO/In2O3 nanocomposites is shown in Fig. 4b. Under the atmosphere of different concentrations of NH3, the sensor response increased approximately linearly during the gas concentration ranging from 100 ppm to 1000 ppm (log plot). To investigate the selectivity of rGO/In2O3 nanocomposites, gas sensors were exposed to seven different gases towards 100 ppm at room-temperature (Fig. 4c). By contrast with the sensitivity towards NH3, the response to other six gases is very weak. Therefore, gas sensors based on rGO/In2O3 nanocomposites possess a prominent selectivity towards NH3 gas. According to the results of gas sensing measurements, the rGO/In2O3 nanocomposites have prominent room-temperature NH3-sensing properties and the reason can be depicted by two aspects (Fig. 4d). On the one hand, the In2O3 nanocubes were composited of nanoparticles. With the decomposion of organic lands and the porous nanostructures are beneficial for the gas diffusion and adsorption. Meanwhile, the high specific surface area of samples will provide more activated sites and promote the gas adsorption [29-31]. On the other hand, the heterointerfaces between In2O3 nanocubes and rGO will be another motivator for the enhanced sensing performances. With a few amount introduced in samples, rGO can be dispersed adequately and there is less physically connection between other rGO sheets. Therefore, a large plenty of interfaces between In2O3 nanocubes and rGO will be formed, which facilitates the electrons transfer between them. The existence of p-n heterojunction can also provide more active sites such as point defects and vacancies, which is one of the key points for the improving sensors performances [32, 33]. From above discussion, the existence of rGO can really promote sensors properties due to the formation of p-n heterojunction.
|
Download:
|
| Fig. 4. (a) Response and recovery curves of sensor based on rGO/In2O3 nanocomposites to 100 ppm NH3 at room-temperature. (b) The dependence of the sensor response on NH3 gas concentration based on log plot in a range of 100-1000 ppm. (c) Sensor responses to different gases (100 ppm) of rGO/In2O3 nanocomposites at room-temperature. (d) Schematic diagram for possible gas sensing mechanisms of rGO/In2O3 nanocomposites. | |
In conclusion, rGO/In2O3 nanocomposite consisting of In2O3 nanocubes uniformly anchored on rGO is developed. Sensor based on rGO/In2O3 nanocomposite shows high response and selectivity to NH3 gas at room temperature, which is ascribed to the porous nanostructures and heterojunction.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 61102006) and Natural Science Foundation of Shandong Province, China (Nos. ZR2015EM019 and ZR2014EL006).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.01.025.
| [1] |
Q.X. Feng, Y.M. Zeng, P.C. Xu, et al., J. Mater. Chem. A 7 (2019) 27522-27534. DOI:10.1039/C9TA11550A |
| [2] |
M. D'Arienzo, L. Armelao, C.M. Mari, et al., J. Am. Chem. Soc. 133 (2011) 5296-5304. DOI:10.1021/ja109511a |
| [3] |
A. Kolmakov, D.O. Klenov, Y. Lilach, et al., Nano Lett. 5 (2005) 667-673. DOI:10.1021/nl050082v |
| [4] |
W.C. Wang, F.Q. Liu, B. Wang, Y.D. Wang, Chin. Chem. Lett. 30 (2019) 1261-1265. DOI:10.1016/j.cclet.2018.12.030 |
| [5] |
L.L. Wang, Z. Lou, T. Fei, T. Zhang, J. Mater. Chem. 22 (2012) 4767-4771. DOI:10.1039/c2jm15342d |
| [6] |
C.Y. Wang, Y.H. Li, P.P. Qiu, et al., Chin. Chem. Lett. 31 (2020) 1119-1123. DOI:10.1016/j.cclet.2019.08.042 |
| [7] |
L.L. Wang, S. Chen, W. Li, et al., Adv. Mater. 31 (2019) 1804583. DOI:10.1002/adma.201804583 |
| [8] |
Q. Zhang, H. Zhang, M.K. Xu, Z.R. Shen, Q. Wei, Chin. Chem. Lett. 29 (2018) 538-542. DOI:10.1016/j.cclet.2017.09.018 |
| [9] |
J.H. Ma, Y. Ren, X.R. Zhou, et al., Adv. Func. Mater. 28 (2018) 1705268. DOI:10.1002/adfm.201705268 |
| [10] |
H. Mbarek, M. Saadoun, B. Bessaïs, Mater. Sci. Eng. C 26 (2006) 500-504. DOI:10.1016/j.msec.2005.10.037 |
| [11] |
K. Wang, J. Li, W. Li, et al., Adv. Mater. Technol. 4 (2019) 1800521. DOI:10.1007/s00339-014-8667-x |
| [12] |
J.W. Ni, T. Zhao, P.C. Xu, et al., Chin. Chem. Lett. 31 (2020) 1680-1685. DOI:10.1016/j.cclet.2019.11.025 |
| [13] |
E.J. Lee, A. VahidMohammadi, B.C. Porok, et al., ACS Appl. Mater. Interfaces 9 (2017) 37184-37190. DOI:10.1021/acsami.7b11055 |
| [14] |
G. Neri, N. Pinna, Angew. Chem. Int. Ed. 51 (2012) 11053-11057. DOI:10.1002/anie.201204373 |
| [15] |
L.J. Zhao, K. Wang, W. Wei, L.L. Wang, W. Han, InfoMat 1 (2019) 407-416. DOI:10.1002/inf2.12032 |
| [16] |
H.Y. Chen, J. Wang, L. MENG, T. Yang, K. Jiao, Chin. Chem. Lett. 27 (2016) 231-234. DOI:10.1016/j.cclet.2015.09.018 |
| [17] |
Z.R. Ma, P. Song, Z.X. Yang, Q. Wang, Appl. Surf. Sci. 465 (2019) 625-634. DOI:10.1016/j.apsusc.2018.09.233 |
| [18] |
D.Z. Zhang, J.J. Liu, H.Y. Chang, A.M. Liu, B.K. Xia, RSC Adv. 5 (2015) 18666-18672. DOI:10.1039/C4RA14611E |
| [19] |
L. Li, S.J. He, M.M. Liu, C.M. Zhang, W. Chen, Anal. Chem. 87 (2015) 1638-1645. DOI:10.1021/ac503234e |
| [20] |
P.A. Russo, N. Donato, S.G. Leonardi, et al., Angew. Chem. Int. Ed. 51 (2012) 11053-11057. DOI:10.1002/anie.201204373 |
| [21] |
Q.X. Feng, X.G. Li, J. Wang, A.M. Gaskov, Sens. Actuator. B-Chem. 222 (2016) 864-870. DOI:10.1016/j.snb.2015.09.021 |
| [22] |
S.Z. Deng, V. Tjoa, H.M. Fan, et al., J. Am. Chem. Soc. 134 (2012) 4905-4917. DOI:10.1021/ja211683m |
| [23] |
X.G. Li, Y.Y. Zhao, X.Y. Wang, et al., Sens. Actuator. B-Chem. 230 (2016) 330-336. DOI:10.1016/j.snb.2016.02.069 |
| [24] |
J. Zhang, J.J. Wu, X.X. Wang, D.W. Zeng, C.S. Xie, Sens. Actuator. B-Chem. 243 (2017) 1010-1019. DOI:10.1016/j.snb.2016.12.062 |
| [25] |
G. Neri, S.G. Leonardi, M. Latino, et al., Sens. Actuator. B-Chem. 179 (2013) 61-68. DOI:10.1016/j.snb.2012.10.031 |
| [26] |
S. Zhang, P. Song, Z.B. Tian, Q. Wang, Mater. Sci. Semicon. Proc. 75 (2018) 58-64. DOI:10.1016/j.mssp.2017.11.029 |
| [27] |
P. Song, D. Han, H.H. Zhang, et al., Sens. Actuator. B-Chem. 196 (2014) 434-439. DOI:10.1016/j.snb.2014.01.114 |
| [28] |
A. Tricoli, M. Righettoni, A. Teleki, Angew. Chem. Int. Ed. 49 (2010) 7632-7659. DOI:10.1002/anie.200903801 |
| [29] |
X.R. Zhou, X.W. Cheng, Y.H. Zhu, et al., Chin. Chem. Lett. 29 (2018) 405-416. DOI:10.1016/j.cclet.2017.06.021 |
| [30] |
X.S. Liang, T.H. Kim, J.W. Yoon, C.H. Kwak, J.H. Lee, Sens. Actuator. B-Chem. 209 (2015) 934-942. DOI:10.1016/j.snb.2014.11.130 |
| [31] |
J.H. Lee, Sens. Actuator. B-Chem. 140 (2009) 319-336. DOI:10.1016/j.snb.2009.04.026 |
| [32] |
D.R. Miller, S.A. Akbar, P.A. Morris, Sens. Actuator. B-Chem. 204 (2014) 250-272. DOI:10.1016/j.snb.2014.07.074 |
| [33] |
P. Ding, D.S. Xu, N. Dong, et al., Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2019.11.024.
|
 2020, Vol. 31
2020, Vol. 31 

