b GRIMAT Engineering Institute Co., Ltd., Beijing 101407, China
Alcohols, especially methanol and ethanol, are widely used chemical reagent in chemical industry, food industry and transportation safety. Nevertheless, they are flammable and explosive [1]. Therefore, accurate and timely detection of alcohol leakage has an important impact on ensuring social safety and quality of life.
Semiconductor metal oxide sensors (MOS sensor) are widely used in gas detection (e.g., ethanol, acetone, ammonia) for their advantages such as low cost, good sensitivity [2]. However, MOS sensors suffer from high operating temperature. Metal oxide material is one of the key factors affecting the performance of gas sensors. A large number of scientific studies have shown that ntype metal oxide materials are widely used in gas sensors [3-8]. For example, SnO2-and WO3-based sensors are commercial gas sensor. Li et al. [3] prepared porous SnO2 nanowires by electrospinning followed with hydrothermal etching treatment and its response value to 100 ppm ethanol is 17.0 at 380 ℃. Ambardekar et al. [4] prepared SnO2 thin film by using an atmospheric plasma spray and its response to 300 ppm ethanol at 300 ℃ is 1.84. In addition to above-mentioned SnO2 material, ZnO is another commonly used ntype semiconductor material in alcohol detection. Li et al. [5] synthetized ZnO nanocages with larger specific surface area by pyrolysis of Zn-based MOFs and it showed high chemical sensing properties towards ppb or sub-ppm level VOC gases. Zhu et al. [6] synthesized ZnO nanoparticles, nanoplates and nanoflowers by hydrothermal route. Among them, ZnO nanoflowers showed the highest response value to 400 ppm ethanol at 350 ℃. However, although these n-type semiconductor oxides have advantage of high sensitivity, their operating temperature is relatively high.
The p-type semiconductor metal oxides, such as Co3O4, are another type of effective gas sensing material. These materials have lower operating temperature, which means lower energy consumption, and it is important for smart electronics and internet of things (IOT) applications. For example, Li et al. [9] synthesized flower-like Co3O4 nanostructures supported on carbon foam (Co3O4@CF) by hydrothermal-annealing route. Its response value to 100 ppm ethanol at 320 ℃ is about 4.2. Ma et al. [10] prepared Co3O4 nanowires via modified template method and it showed the response value about 5.1–300 ppm ethanol at 350 ℃. Sun et al. [11] fabricated Co3O4 nanocubes via a microwave-assisted solvothermal process and its response to 100 ppm ethanol is 5.0 at 200 ℃. Li et al. [12] fabricated 2D ultrathin Co3O4 nanosheet on 3D carbon foam and the response value is 10.4 for 100 ppm ethanol at 100 ℃. Table S1 (Supporting information) listed the ethanol sensing performance of different gas sensor reported in the literatures.
Herein, a homogeneous ZnO-Co3O4 bimetallic oxide (named as Zn-CoOx), which shows p-type semiconductor properties, was prepared by a facile coprecipitation-annealing route.
In a typical process, 4 mmol cobaltous nitrate (99%, Aladdin BioChem Technology Co., Ltd.)and 2 mmol zinc sulphate(99.5%, Aladdin Bio-ChemTechnology Co., Ltd.)were dissolved ina mixed solutionof 20 mL deionized water and 20 mL ethanol. Then, 24 mmol 2-methylimidazole (98%, Aladdin Bio-Chem Technology Co., Ltd.) was dissolved in 20 mL each of deionized water and ethanol. Later, the metal salt solution was dropwise to the precipitant solution and formed precipitation as precursor. The precursor was washed by ethanol and dried at 60 ℃ for 12 h. Finally, the precursor was annealed at 400 ℃ with a heating rate of 2 ℃/min and Zn-CoOx materials were obtained. To investigate the effect of Co content on gas sensing properties, the final samples were obtained by adjusting the Co/Zn feed molar ratio and they were named as Co66Zn34Ox, Co50Zn50Ox, Co34Zn66Ox and Co0Zn100Ox, respectively.
The component of the samples was characterized by X-ray diffraction (XRD, Rigaku D/max-2500, Japan) with the 2θ ranging from 15° to 90°. TG analysis was carried out with a Mettler TGA/DSC 3 thermalanalyzer. The scanning electron microscopy (SEM, Hitachi S-4800, Japan) and energy-dispersive X-ray spectroscopy (EDS, HORIBA EMAX-350, Japan) was tested to confirm the structure and composition. Sensing performance of Zn-CoOx was performed on JF02 F sensing analysis system (Guiyan Jinfeng Technology Co., Kunming, China). In a typical test process, the samples were loaded on ceramic substrates as simple sensors. Then, the test was carried out to confirming the optimal operating temperature, response values, stability and selectivity of the samples. Especially, the response value is defined as the S = (Rg/Ra-1) 100%, where Rg is the stable resistance of the sensor exposed tothe target gas and Ra is the resistance of the sensor in air. The detail test process is listed in Supporting information.
The alcohol detective performance of Zn-CoOx was tested by alternately exposing samples to ethanol vapor and air. The operating temperature is a key factor for sensing performance by influencing the active energy of gas molecular. Fig. 1a shows the response values of Zn-CoOx at different operating temperature (250-450 ℃). It is clearly that Co50Zn50Ox shows the highest response values. However, the resistance of Co50Zn50Ox in 100 ppm ethanol vapor is too high to be detected and the best operating temperature is also too high (400 ℃). Moreover, the response signal of Co50Zn50Ox cannot be detected when the operating temperature is lower than 350 ℃. Thus, we try to change the Co/Zn molar ratio for overcoming these shortcomings. According to Fig. 1a, the response of Co34Zn66Ox is much lower than Co66Zn34Ox, while the optimal operating temperature of Co0Zn100Ox is so high (~450 ℃). The different response value is related to many factors such as porous structure, material properties and operating temperature [13]. The composition of the samples is different, and the substrate material is Co3O4. The continuity main phase is ensured by the higher Co content as well as the binding of Co and ligands. However, n-type semiconductor shows opposite properties of p-type semiconductor. Thus, appropriate Co/Zn ratio is important. We further list the resistances of Zn-CoOx samples in air at optimum operating temperature (Table 1), and it is clearly that resistance rises with the increasing of Zn content. The Co66Zn34Ox give the lowest resistance and optimal operating temperature. Obviously, the absolute optimal operating temperature of the Co66Zn34Ox is higher than some sensing material [14, 15]. The relative higher operating temperature might be related to the dense and stacked structure, which may be caused by the solvent water. However, the optimal operating temperature of Zn-CoOx decreases with the increasing of Co/Zn ratio, indicating the beneficial of composite. In addition, the initial resistance of the Zn-CoOx also decreases with the increasing of Co/Zn ratio. Thus, in order to decrease the resistance and operating temperature, further investigation was carried out on Co66Zn34Ox at the optimal operating temperature of 300 ℃.

|
Download:
|
| Fig. 1. (a) Operating temperature dependence to response of the Co0Zn100Ox, Co34Zn66Ox, Co50Zn50Ox and Co66Zn34Ox to 100 ppm ethanol at temperature ranges from 250 ℃ to 450 ℃. (b) Dynamic sensing characteristics (Inset: liner relation between response to concentration) of the Co66Zn34Ox to different ethanol concentration at 300 ℃. (c) Stability curve of Co66Zn34Ox (Inset: dynamical responses to 100 ppm ethanol at 300 ℃). (d) Selectivity of Co66Zn34Ox to 10 ppm various reductive gases (methane, hydrogen, ammonia and methanol) at 300 ℃. | |
|
|
Table 1 The resistances of Zn-CoOx in air and its response to 100 ppm ethanol at optimum operating temperature. |
Sensing performance of Co66Zn34Ox at different ethanol concentrations was evaluated. Fig. 1b shows the dynamic curve of Co66Zn34Ox, in which the ethanol vapor concentration changes from 5 ppm to 500 ppm. In a response and recovery cycle, the response of Co66Zn34Ox firstly increases rapidly achieving an almost stable state and then increases slowly when Co66Zn34Ox exposed to ethanol. When the sensor removes back to air, the response of Co66Zn34Ox decreases. The inset of Fig. 1b is the relationship between response value and ethanol concentration. Evidently, the response value of Co66Zn34Ox is good linear within ethanol concentration range of 5–100 ppm. Additionally, the detection limit is calculated as 0.243 ppm by setting detection criterion as 3 times as standard deviation. Furthermore, the response and recovery time is a little longer than some sensing material [12, 16]. The reasons involve temperature, structure, etc. The high initial resistance leads to a higher operating temperature. However, the response time is positive related to temperature [17]. The response time will rise with the increasing of temperature. As for recovery process, it is a complex process. The recovery time is determined by the temperature and adsorption/desorption of gas molecules [13]. Higher temperature is beneficial to gas desorption and diffusion. However, the adsorption and desorption process are opposite process. The competition of the process will increase recovery time. In addition, the agglomeration of the material unit is not conducive to diffusion. Thus, the base line is hard to return to initial state and the baseline is increasing in the test process.
The stability and selectivity of Co66Zn34Ox were also tested. Fig. 1c shows the long-stability curve of Co66Zn34Ox. It is obviously that the Co66Zn34Ox maintains good stability after 28 days, suggesting the durability of the sensor. Besides, the inset of Fig. 1c is the dynamical response of Co66Zn34Ox to 100 ppm ethanol vapor. The dynamic curves are almost at the same level after six continuous cycles, indicating good repeatability of the sensor. Fig. 1d is the Co66Zn34Ox response to various reductive gases (methane, hydrogen, ammonia and methanol), which are often coexist with ethanol. The response value of Co66Zn34Ox to ethanol (290.4%) and methanol (283.5%) is better than those to methane (101.6%), hydrogen (154.4%), ammonia (57.3%).
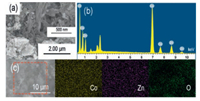
The microstructure morphology of Co66Zn34Ox and other Zn-CoOx are observed by SEM, EDS and element mapping (Fig. 2 and Fig. S2 in Supporting information). It is clearly that Co66Zn34Ox owns interconnected porous structure with agglomeration, in which the basic constitution diameter is 150-200 nm (Fig. 2a). Fig. 2b is the EDS spectrum of Co66Zn34Ox, and it is obviously that Co66Zn34Ox consists of Co and Zn without impurity atoms and the molar ratio of Co/Zn is about 3.4. Fig. 2c is the element mapping of Co66Zn34Ox, and Co, Zn and O is distributed uniformly, suggesting uniformly formation of porous Zn-Co bimetallic oxides.
|
Download:
|
| Fig. 2. (a) SEM image, (b) EDS spectrum and (c) element mapping of Co66Zn34Ox. | |
The XRD pattern of the Co66Zn34Ox is shown in Fig. 3a. The peaks around 2u = 19.3°, 31.6°, 37.1°, 59.5° and 65.4° are indexed to (111), (220), (311), (511) and (440) planes of cubic Co3O4 (JCPDS No.42-1467). The peaks at (2θ=) 31.7° and 55.8° indexed to (100) and (110) planes of hexagonal ZnO (JCPDS No. 36-1451). It is obviously that all diffraction peaks are well in agreement with the standard card of Co3O4 and ZnO, indicating the conversion of Zn-Co precursor. In addition, there are no impurity peaks in the pattern, demonstrating purity of the Co66Zn34Ox and the results are consistent with EDS results.

|
Download:
|
| Fig. 3. (a) XRD patterns of Co66Zn34Ox. (b) TG curves of Zn-CoOx. | |
The TG curves of the samples are shown in Fig. 3b. Obviously, slight weight loss is observed under 300 ℃ of Co66Zn34Ox, corresponding to the remove of water vapor and solvent molecules. Then, the precursor undergoes gradual decrease in weight between 300 ℃ and 400 ℃. On the one hand, this observation confirmed that the precursor was partial decomposed. On the other hand, it indicates that the porous structure of the sample was kept. A drastic weight decrease is observed between 400 ℃ and 500 ℃. The weight remains at 45% of the original weight when the calcination temperature further increases, indicating the complete decomposition of the precursor and the collapse of structure. Other Zn-CoOx samples show the similar thermogravimetric characteristics, while Co0Zn100Ox shows drastic weight loss between 200 ℃ and 650 ℃.
According to the dynamic sensing curve, the Co66Zn34Ox is a typical p-type semiconductor material with Co3O4 as substrate material. In general, the sensing mechanism is based on gas adsorption and desorption and adsorbed oxygen molecules are key factors for sensing performance. When the sensor exposed in air, the oxygen molecules are adsorbed on surface of material. In this process, the adsorption oxygen molecules will capture electrons from Co66Zn34Ox, and thus form ionizing oxygen. In addition, the adsorption oxygen molecules contain three types, that is O2-, O- and O2-, due to the different temperature [18]. The process is displayed as follows (Eqs. 1–4):
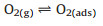
|
(1) |

|
(2) |
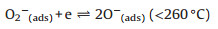
|
(3) |
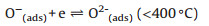
|
(4) |
When the sensor sealed to alcohol vapor, the ionized oxygen will reactwith targetgas and form CO2 and H2O. In this process, the electrons will be released and neutralized with holes, and thus resistance will increase [14, 19]. The process is follows (Eqs. 5–7):

|
(5) |
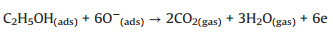
|
(6) |
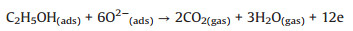
|
(7) |
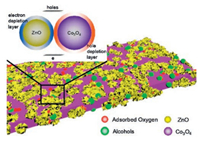
In addition to the above mechanism, p-n heterojunction will be formed on the surface between Co3O4 and ZnO. The band gap of Co3O4 and ZnO are different, and thus the electrons will flow from ZnO to Co3O4 until the Fermi level achieving equalization. In this process, electron depletion layer and hole depletion layer will be formed on the interface of ZnO and Co3O4, respectively. When the sensor exposed in air, the electron density decreases, and the electron transferring also decreases. The resistance thus maintains a relatively lower state because depletion layer become thinner. When the sensor exposed in alcohol vapor, the electron density increases, and more electrons transfer from ZnO to Co3O4. Thus, the resistance further increases due to the thicker depletion layer and sensing performance is improved (Fig. 4) [20, 21].
|
Download:
|
| Fig. 4. Schematic diagram of Co66Zn34Ox. | |
In summary, porous Zn-CoOx was fabricated by precipitationannealing route. The ratio of Co to Zn of Zn-CoOx could be controlled from 2.0 to 0.5. Interestingly, the optimal operating temperature of the Zn-CoOx was decreased with the increasing of the Co/Zn feed ratio. In particular, the Co66Zn34Ox showed good gas sensing response value to 100 ppm ethanol at 300 ℃, in which the Co50Zn50Ox was excluded because higher resistances. The sensing performance observed here might be related to the p-n heterojunction formed between Co3O4 and ZnO, in which the space charge layer greatly contributed to the material resistance changing in air and ethanol. Overall, this study will promote the development of gas sensor with low operating temperature and resistance.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis work was supported by National Natural Science Foundation of China (No. 61874137).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.01.012.
| [1] |
S.Y. Kim, J. Kim, W.H. Cheong, et al., Sens. Actuator. B: Chem. 259 (2018) 825-832. DOI:10.1016/j.snb.2017.12.139 |
| [2] |
C. Han, X. Li, C. Shao, et al., Sens. Actuator. B: Chem. 285 (2019) 495-503. DOI:10.1016/j.snb.2019.01.077 |
| [3] |
R. Li, S. Chen, Z. Lou, et al., Sens. Actuator. B: Chem. 252 (2017) 79-85. DOI:10.1016/j.snb.2017.05.161 |
| [4] |
V. Ambardekar, P.P. Bandyopadhyay, S.B. Majumder, J. Alloys. Compd. 752 (2018) 440-447. DOI:10.1016/j.jallcom.2018.04.151 |
| [5] |
W. Li, X. Wu, N. Han, et al., Sens. Actuator. B: Chem. 225 (2016) 158-166. DOI:10.1016/j.snb.2015.11.034 |
| [6] |
L. Zhu, Y. Li, W. Zeng, Appl. Surf. Sci. 427 (2018) 281-287. DOI:10.1016/j.apsusc.2017.08.229 |
| [7] |
Y. Zhang, J. Xu, Q. Xiang, et al., J. Phys. Chem. 113 (2009) 3430-3435. DOI:10.1021/jp8092258 |
| [8] |
X. Zhang, B. Liu, Y. Xu, et al., CrystEngComm 21 (2019) 7528-7534. DOI:10.1039/C9CE01530B |
| [9] |
L. Li, M. Liu, S. He, W. Chen, Anal. Chem. 86 (2014) 7996-8002. DOI:10.1021/ac5021613 |
| [10] |
M.X. Ma, Z.Y. Pan, L. Guo, et al., Chin. Sci. Bull. 57 (2012) 4019-4023. DOI:10.1007/s11434-012-5363-0 |
| [11] |
C. Sun, X. Su, F. Xiao, C. Niu, J. Wang, Sens. Actuator. B: Chem. 157 (2011) 681-685. DOI:10.1016/j.snb.2011.05.039 |
| [12] |
L. Li, C. Zhang, R. Zhang, et al., Sens. Actuator. B: Chem. 244 (2017) 664-672. DOI:10.1016/j.snb.2017.01.056 |
| [13] |
X. Zhou, X. Cheng, Y. Zhu, et al., Chin. Chem. Lett. 29 (2018) 405-416. DOI:10.1016/j.cclet.2017.06.021 |
| [14] |
F.I. Shaikh, L.P. Chikhale, I.S. Mulla, S.S. Suryavanshi, Powder Technol. 326 (2018) 479-487. DOI:10.1016/j.powtec.2017.12.028 |
| [15] |
B. Behera, S. Chandra, Sens. Actuator. B: Chem. 229 (2016) 414-424. DOI:10.1016/j.snb.2016.01.079 |
| [16] |
F. Meng, N. Hou, S. Ge, et al., J. Alloys. Compd. 626 (2015) 124-130. DOI:10.1016/j.jallcom.2014.11.175 |
| [17] |
J.D. Prades, R. Jimenez-Diaz, F. Hernandez-Ramirez, et al., Appl. Phys. Lett. 93 (2008) 123110. DOI:10.1063/1.2988265 |
| [18] |
C. Jin, S. Park, H. Kim, C. Lee, Sens. Actuator. B: Chem. 161 (2012) 223-228. DOI:10.1016/j.snb.2011.10.023 |
| [19] |
Y. Chen, H. Li, Q. Ma, et al., Appl. Surf. Sci. 439 (2018) 649-659. DOI:10.1016/j.apsusc.2018.01.084 |
| [20] |
Y. Xiong, W. Xu, Z. Zhu, et al., Sens. Actuator. B: Chem. 253 (2017) 523-532. DOI:10.1016/j.snb.2017.06.169 |
| [21] |
B. Li, J. Liu, Q. Liu, et al., Appl. Surf. Sci. 475 (2019) 700-709. DOI:10.1016/j.apsusc.2018.12.284 |
 2020, Vol. 31
2020, Vol. 31 


