b Indian Institute of Technology Guwahati, Guwahati 781039, India
Tetracycline (TC), as a typical broad-spectrum antibiotic, has been widely employed in veterinary, agricultural and humanic therapeutics. However, only little amount of TC can be metabolized by humans and animals owing to its chemical structure and recalcitrance to biological degradation, which results in the massive release of its residues into the environment, affecting the ecosystem. Therefore, the development of an alternative technology to efficiently remove TC is urgent [1-3].
Based on hydrogen peroxide (H2O2) and ferrous ions, the Fenton process has recently garnered tremendous attention as an efficient approach to degrade organic pollutants via the hydroxyl radical (·OH) oxidation [4-9]. Whereas, narrow work pH range and the low utilization rate of H2O2 significantly limit its applications in actual water treatment [10, 11]. As an alternative, through the activation of peroxymonosulfate (PMS), the sulfate radical (SO4·-) based Fenton-like system [12, 13] has recently beenwidely investigated due to it not only shows higher oxidation potentials (SO4·-(2.5–3.1 V) > ·OH (1.8– 2.7 V)) but also works in wide pH ranges [14, 15]. Till now, various strategies such as base, heat, ultrasound transition metals and ultraviolet (UV) have been adopted to active PMS [16-18]. Among them, the utilization of transition-metal ions such as Mn2+, Co2+ and Cu2+, are of great significance owing to its high efficiency and abundant resources [19-21]. But during application such as in a slurry operating system, they are especially arduous to be separated and regenerated. Separation can be enhanced in the form of particles, but the catalytic efficiency was reduced owing to the decrease of active sites. Therefore, the development of a catalyst for degradation of TC and activation of PMS with simultaneous facile separation and high efficiency is desirable.
Recently, yolk-shell structured mesoporous materials have shown great promise in diverse applications such as drug delivery [22, 23], sensors [24-26], energy storage [27], confined catalysis [28-30] and adsorption [31, 32] due to the unique structure of inner active core, middle void spaces, and outer mesoporous shells. Among them, the Fe3O4@void@mesoporous SiO2 (mSiO2) nanostructures are of great interest due to their superior properties such as strong magnetic separation ability, outstanding biocompatibility, abundant catalytic sites, high specific surface area and multi-functional interfaces [33-35]. However, the small magnetization of the resultant materials still hinders their practical applications. The utilization of Fe, Co, Ni or alloys metal cores [36, 37] may resolve this problem, but they are easy to suffer from aggregation and the surface of them are hard to be functionalized during the synthesis, which are undesirable for the further coating. Consequently, the development of a facile method to synthesize Fe@void@mesoporous SiO2 (mSiO2) is much desired.
Herein, we have developed a facile strategy to prepare magnetic yolk-shell structured mesoporous silica nanospheres (Fe@void@mSiO2). The synthesis involved a successive coating strategy, followed by a high-temperature in-situ transform of Fe3O4 to Fe nanoparticles. The introduction of a middle carbon layer between mesoporous silica shell and Fe3O4 core is to in-situ reduce Fe3O4 to Fe and generate void space during the carbonization process. The resultant materials show a well-defined yolk–shell structure with high specific surface area (495.0 m2/g), uniform pore size (6.9 nm) and super large magnetic susceptibility (105 emu/g). Finally, dedicated to the activation of PMS, the material used as a catalyst shows an excellent degradation activity for TC in a wide pH range. Moreover, the catalyst is able to be fast recycled via applying an external magnetic field, which holds a great promise in the practical application.
All chemicals without further purification were used as received. Millipore water was suitable for all experiments. FeCl3·6H2O, PMS (KHSO5·0.5KHSO4·0.5K2SO4), tetraethyl orthosilicate (TEOS), ethanol, methanol, cyclohexane, trisodium citrate, ammonia solution (28 wt%), ethylene glycol, sodium acetate, hexadecyl trimethyl ammonium bromide and TCL were purchased from Shanghai Chemical Corp.
Referring to previous work, a solvothermal method is adopted to the preparation of superparamagnetic Fe3O4 nanoparticles [38-40]. In brief, sodium acetate (NaAc, 6.0 g), trisodium citrate (1.3 g) and FeCl3·6H2O (3.25 g) were added to ethylene glycol (80 mL) with stirring and dissolve. The mixture stirred strongly at room temperature for 1 h was poured into a stainless-steel autoclave lined with Teflon and 100 mL volume. The autoclave was kept for 10 h at 200 ℃, and slowly cooled to room temperature follow. The products obtained were washed for 3 times with EtOH and deionized water, separately, and then dispersed in EtOH (45 mL) for the next step.
Fe3O4 ethanol dispersion (3 mL) was sonicated and dissolved in a mixed solution of deionized water (10 mL) and EtOH (20 mL). Subsequently, formaldehyde (0.10 g, 37 wt%), resorcinol (0.10 g, 0.09 mmol/L) and ammonia solution (0.50 g, 28 wt%) were continuously added. Formaldehyde polymerization of formaldehyde and resorcinol on Fe3O4 particles occurred in the mixed dispersion after mechanical stirring for 10 h at 45 ℃. Via washed with EtOH and deionized water as well as magnet for three times, separately, the core-shell Fe3O4@RF microspheres were obtained.
A mixed solution containing concentrated aqueous ammonia (0.80 mL, 28 wt%), deionized water (80 mL) and CTAB [41, 42] (0.50 g, 1.3 mmol) was prepared, and then the Fe3O4@RF nanoparticles obtained above were dispersed in them by sonication. Subsequently, a two-phase system was formed with the supplement of cyclohexane (20 mL). Then, TEOS (0.5 mL) was added into the oil phase, and the reaction was maintained at 45 ℃ with slow agitation (100 rpm) for 6 h [43]. The product collected by the magnet was washed three times with EtOH and water, respectively. Finally, the microspheres were obtained by calcination in N2 atmosphere at 850 ℃.
Transmission electron microscopy (TEM) images was taken on a JEOL 2011 microscope (Japan). Prior to TEM measurements, the sample was dispersed in EtOH and dropped on a holey carbon film on a Cu grid. Vibrating Sample Magnetometer (EV9, Microsense, Japan) was used to measure the magnetization. XRD patterns were obtained on a Bruker D8X-ray diffractometer with Ni-filtered Cu Kα radiation (40 kV, 40 mA). Nitrogen sorption isotherms were measured with a Micromeritics Tristar 3020 analyzer (USA) at 77 K. Before the measurements, the samples were degassed in a vacuum at 180 C for 6 h. The specific surface areas (SBET) was calculated using adsorption data in the relative pressure range P/P0 = 0.05–0.3 based on Brunauer-Emmett-Teller (BET) method. The Barrett-Joyner-Halenda (BJH) model was applied to estimate the pore size distributions from the adsorption branches of the isotherms. The total pore volumes were calculated from the adsorbed amount at the relative pressure P/P0 = 0.995.
Catalyst (0.05 g) and aqueous suspensions (100 mL) of TC (10 mg/L) were added into a double-layered cylindrical reactor (100 mL in capacity). The utilization of a water jacket maintains the temperature of the solution at 20 ℃. The mixture was mechanically stirred for 30 min in dark before the addition of PMS (0.18 g) to achieve an adsorption/desorption equilibrium between the contaminants and the catalyst. Then, the suspension (0.5 mL) was removed through the utilization of a 1 mL syringe at given time intervals and was filtered via a membrane with a pore size of 0.45 μm. Moreover, methanol (2 mL) was added to the sample above to terminate the reaction. The mixed solution was analyzed by recording the variations in the maximum absorption band (405 nm) using a Thermo Spectronic UV 500 UV–vis spectrometer.
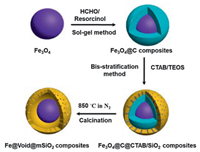
Fig. 1 shows illustration of the formation of yolk-shell structured Fe@void@mSiO2 composites by a facile and straightforward high temperature in-situ reduction process. First, uniform Fe3O4 nanoparticles were prepared via a modified solvothermal process and then encapsulated with a resorcinol formaldehyde (RF) polymer layer through a sol-gel method, giving rise to coreshell structured Fe3O4@RF composites. Then, a biphase stratification method was adopted to coat CTAB/SiO2 composite layer onto the surface of Fe3O4@C composites, which leads to Fe3O4@C@C-TAB/SiO2 composites. Finally, uniform yolk-shell structured Fe@void@mSiO2 composites were obtained after calcination in N2 at 850 ℃.
|
Download:
|
| Fig. 1. Illustration of the formation of yolk-shell structured Fe@void@mSiO2 composites. | |
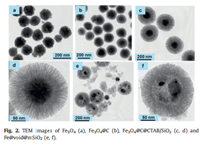
TEM image (Fig. 2a) possesses that the hydrothermally synthesized Fe3O4 nanoparticles display well-dispersed morphology with a uniform particle size of ~120 nm. After the polymerization, a thin RF polymer layer of ~30 nm was successfully wrapped on the Fe3O4 nanoparticles (Fig. 2b). After deposition of a layer of mesoporous silica through the oil-water biphase stratification method, Fe3O4@C@CTAB/SiO2 composites with discrete and regular spherical morphology can be formed (Fig. 2c). TEM images show that the Fe3O4@C@CTAB/SiO2 composites possess a well-defined core-shell-shell structure with a layer of mesoporous silica in 40 nm thickness (Fig. 2d). After the in-situ high temperature reduction process, the well-defined core-shell structure was transformed to yolk-shell structure (Figs. 2e and f) with a number of nanoparticles broken, which is probably due to the recrystallization of Fe.
|
Download:
|
| Fig. 2. TEM images of Fe3O4 (a), Fe3O4@C (b), Fe3O4@C@CTAB/SiO2 (c, d) and Fe@void@mSiO2 (e, f). | |
The wide-angle X-ray diffraction (XRD) pattern of Fe@void@m-SiO2 composites (Fig. 3a) displayed several sharp which can be assigned to iron nanoparticles while they were not observed in Fe3O4. The wide peaks at 2θ = 22° and 26° can be assigned to amorphous mesoporous silica and the graphitized carbon, respectively. Two broad peaks located at 1350 and 1590 cm -1 in the Raman spectra are attributed to the D- and G- bands, respectively (Fig. 3b). The hysteresis loop data suggests that our material possess a quite high magnetization of 105 emu/g, which is much stronger than Fe3O4 (56 emu/g, Fig. 3c). Nitrogen adsorption-desorption isothermals show a typical type-IV curve with distinct hysteresis loops within the relative pressure band of 0.45-0.80, indicating the uniform mesoporous structure (Fig. 3d). The BET surface area of the Fe@void@mSiO2 composites are calculated to be 495 m2/g. The pore size distribution derived from the adsorption branch using the BJH method shows that the corresponding pore size is centered at 6.9 nm, making them an ideal candidate for the later applications.

|
Download:
|
| Fig. 3. (a) XRD patterns, (b) Raman spectra, (c) the magnetic hysteresis loops and (d) N2 Sorption isotherms of the resultant materials. | |
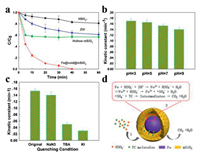
The performance of the resultant magnetic mesoporous silica on the activation of PMS for the degradation of TC was examined at pH of 7 (Fig. 4a). As a comparison, the degradation performances of TC under PMS and ZVI were also examined, exhibiting the removal efficiencies of 19% and 39%, respectively in 60 min. When Fe@void@mSiO2 nanospheres were used as the catalyst, the removal efficiency reaches to 100% in 40 min, clearly demonstrating the priority of Fe@void@mSiO2 nanospheres. The effect of pH on the degradation performance of Fe@void@mSiO2 (Fig. 4b) was investigated. In order to estimate the kinetic constant, a pseudo-first-order kinetic model was employed (Eq. 1).
|
Download:
|
| Fig. 4. (a) Degradation profile of different catalysts and (b) kinetic constants values at different pH. Experimental conditions: solution volume: 100 mL, catalyst dose: 0.1 g/L, PMS concentration: 0.18 g/L, T: 20 ℃, initial TC concentration: 10 mg/L. (c) Scavenging test and (d) schematic illustration of the possible degradation mechanism. | |
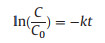
|
(1) |
where C is the concentration of TC at time t, C0 is the initial TC concentration and k is the pseudo-first-order reaction rate constant. We caught sight of the kinetic constant at pH of 7 and 9 are calculated to be 0.15 and 0.14 min -1, respectively, slightly smaller than that at pH of 3, suggesting that such catalysts are able to work over a broad pH range. As illustrated in Fig. 4c, the production of ·OH/SO4·- as the dominate species in this systemwas proved through the scavenging test. The addition of NaN3 (singlet oxygen (1O2) scavenger) slightly reduced the reaction rate, indicating that 1O2 is not the main species while the addition of tert-butanol significantly reduced the degradation rate, suggesting that ·OH/SO4·- are the main contributor. Note that by the addition of KI, which is a scavenger for the surface bonded radicals, the reaction rate sharply reduced, suggesting that surface radicals play the most important role in the degradation process. Magnetic mesoporous silica exhibits extraordinary performance, which can be ascribed to the synergistic effect from its unique textual structures. Firstly, the ordered large mesopores facilitates the mass transfer of both PMS and TC molecules between solid and phases aqueous. Secondly, the presence of mesoporous silica shell greatly enhances the adsorption of pollutants molecules and enrichs them in the void space of the nanoreactor, which could be beneficial for surface reaction [44]. The adsorption test obviously shows that the hollow structured mesoporous silica shell possesses a high removal efficiency for TC (~40%). Finally, the surface iron species reacts with the adsorbed PMS to produce large quantity of radicals such as ·OH/SO4·-, which can directly in-situ oxidize the TC molecules confined in the void space into small molecules or even CO2 and H2O (Fig. 4d).
In summary, a successive coating strategy followed by a subsequent high-temperature in-situ treatment have been employed for the preparation of yolk-shell structured Fe@void@m-SiO2 composites. The obtained composites exhibit a uniform pore size (~6.9 nm), a high specific surface area (~495.0 m2/g) and a super large magnetic susceptibility (~105 emu/g), which is suitable for the activation of PMS to degrade organic pollutants. Compared with commercial ZVI nanoparticles, the composite catalyst possesses much more excellent degradation activity for tetracycline (TC) in a wide pH range and rapid recovery via using an external magnetic field. This study paves a new strategy to the diversification of catalysts for the Fenton-like process.
Declaration of competing interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research is supported by the NSF of China (Nos. 51822202 and 51772050), Shanghai Rising-Star Program (No. 18QA1400100), Youth Top-notch Talent Support Program of Shanghai, Science and Technology Commission of Shanghai Municipality (No. 19520713200), Shanghai Scientific and Technological Innovation Project (No. 19JC1410400), DHU Distinguished Young Professor Program and Fundamental Research Funds for the Central Universities.
| [1] |
X. Wen, Z. Zeng, C. Du, et al., Chemosphere 222 (2019) 865-871. DOI:10.1016/j.chemosphere.2019.02.020 |
| [2] |
J. Cao, Z. Xiong, B. Lai, Chem. Eng. J. 343 (2018) 492-499. DOI:10.1016/j.cej.2018.03.036 |
| [3] |
J. Hu, R. Yang, Z. Li, et al., Solid State Sci. 92 (2019) 60-67. DOI:10.1016/j.solidstatesciences.2019.02.009 |
| [4] |
J.X. Fan, M.Y. Peng, H. Wang, et al., Adv. Mater. 31 (2019) 1808278. DOI:10.1002/adma.201808278 |
| [5] |
S. Navalon, R. Martin, M. Alvaro, H. Garcia, Angew. Chem. Int. Ed. 49 (2010) 8403-8407. DOI:10.1002/anie.201003216 |
| [6] |
D.Q. He, L.F. Wang, H. Jiang, H.Q. Yu, Chem. Eng. J. 272 (2015) 28-134. DOI:10.1016/j.cej.2015.03.006 |
| [7] |
C. Hu, D. Huang, G. Zeng, et al., Chem. Eng. J. 338 (2018) 432-439. DOI:10.1016/j.cej.2018.01.068 |
| [8] |
M. Cheng, G. Zeng, D. Huang, et al., J. Hazard. Mater. 312 (2016) 184-191. DOI:10.1016/j.jhazmat.2016.03.033 |
| [9] |
X. Yang, X. Cheng, A.A. Elzatahry, et al., Chin. Chem. Lett. 30 (2019) 324-330. DOI:10.1016/j.cclet.2018.06.026 |
| [10] |
L. Chen, J. Ma, X. Li, et al., Environ. Sci. Technol. 45 (2011) 3925-3930. DOI:10.1021/es2002748 |
| [11] |
G.P. Anipsitakis, D.D. Dionysiou, Environ. Sci. Technol. 37 (2003) 4790-4797. DOI:10.1021/es0263792 |
| [12] |
X. Li, X. Huang, S. Xi, et al., J. Am. Chem. Soc. 140 (2018) 12469-12475. DOI:10.1021/jacs.8b05992 |
| [13] |
X. Yang, X. Xu, J. Xu, Y. Han, J. Am. Chem. Soc. 135 (2013) 16058-16061. DOI:10.1021/ja409130c |
| [14] |
W.D. Oh, Z. Dong, T.T. Lim, Appl. Catal. B 194 (2016) 169-201. DOI:10.1016/j.apcatb.2016.04.003 |
| [15] |
W.D. Oh, Z. Dong, G. Ronn, T.T. Lim, J. Hazard. Mater. 325 (2017) 71-81. DOI:10.1016/j.jhazmat.2016.11.056 |
| [16] |
Y. Wang, H. Sun, X. Duan, et al., Appl. Catal. B 172-173 (2015) 73-81. DOI:10.1016/j.apcatb.2015.02.016 |
| [17] |
H. Sun, Y. Wang, S. Liu, et al., Chem. Commun. 49 (2013) 9914-9916. DOI:10.1039/c3cc43401j |
| [18] |
H. Sun, S. Liu, G. Zhou, et al., ACS Appl. Mater. Interfaces 4 (2012) 5466-5471. DOI:10.1021/am301372d |
| [19] |
Y. Yang, Y. Tang, S. Liang, et al., Nano Energy 61 (2019) 617-625. DOI:10.1016/j.nanoen.2019.05.005 |
| [20] |
H. Li, Q. Gao, H. Wang, et al., ACS Omega 3 (2018) 17724-17731. DOI:10.1021/acsomega.8b02577 |
| [21] |
C.X. Zhao, B.Q. Li, J.N. Liu, J.Q. Huang, Q. Zhang, Chin. Chem. Lett. 30 (2019) 911-914. DOI:10.1016/j.cclet.2019.03.026 |
| [22] |
J. Liu, S.Z. Qiao, J.S. Chen, et al., Chem. Commun. 47 (2011) 12578-12591. DOI:10.1039/c1cc13658e |
| [23] |
Y. Chen, H. Chen, D. Zeng, et al., ACS Nano 10 (2010) 6001-6013. |
| [24] |
Z.M. Cui, Z. Chen, C.Y. Cao, L. Jiang, W.G. Song, Chem. Commun. 49 (2013) 2332-2334. DOI:10.1039/c3cc38649j |
| [25] |
C. Liu, J. Li, J. Qi, et al., ACS Appl. Mater. Interfaces 6 (2014) 13167-13173. DOI:10.1021/am503063m |
| [26] |
T. Zhao, Y. Ren, G. Jia, et al., Chin. Chem. Lett. 30 (2019) 2032-2038. DOI:10.1016/j.cclet.2019.05.006 |
| [27] |
T. Zhu, L. Zhu, J. Wang, G.W. Ho, ACS Appl. Mater. Interfaces 8 (2016) 32901-32909. DOI:10.1021/acsami.6b12284 |
| [28] |
Z. Wu, K. Yu, S. Zhang, Y. Xie, J. Phys. Chem. C 112 (2008) 11307-11313. DOI:10.1021/jp803582d |
| [29] |
P. Rai, J.W. Yoon, H.M. Jeong, et al., Nanoscale 6 (2014) 8292-8299. DOI:10.1039/C4NR01906G |
| [30] |
J. Liu, H.Q. Yang, F. Kleitz, et al., Adv. Funct. Mater. 22 (2012) 591-599. DOI:10.1002/adfm.201101900 |
| [31] |
Y. Boyjoo, K. Merigot, J.F. Lamonier, et al., RSC Adv. 5 (2015) 24872-24876. DOI:10.1039/C5RA02427G |
| [32] |
L. Yin, S. Song, X. Wang, et al., Environ. Pollut. 238 (2018) 725-738. DOI:10.1016/j.envpol.2018.03.092 |
| [33] |
P. Qiu, K. Kang, K. Kim, et al., RSC Adv. 5 (2015) 96201-96204. DOI:10.1039/C5RA15693A |
| [34] |
W. Li, D. Zhao, Adv. Mater. 25 (2013) 142-149. DOI:10.1002/adma.201203547 |
| [35] |
T. Yao, T. Cui, X. Fang, et al., Nanoscale 5 (2013) 5896-5904. DOI:10.1039/c3nr01470c |
| [36] |
K.A. Kuttiyiel, Y.M. Choi, K. Sasaki, et al., Nano Energy 29 (2016) 261-267. DOI:10.1016/j.nanoen.2016.05.024 |
| [37] |
K.A. Kuttiyiel, Y.M. Choi, S.M. Hwang, et al., Nano Energy 13 (2015) 442-449. DOI:10.1016/j.nanoen.2015.03.007 |
| [38] |
J. Liu, Z. Sun, Y. Deng, et al., Angew. Chem. Int. Ed. 48 (2009) 5875-5879. DOI:10.1002/anie.200901566 |
| [39] |
B. Thokchom, P. Qiu, M. Cui, et al., Ultrason. Sonochem. 34 (2017) 262-272. DOI:10.1016/j.ultsonch.2016.05.030 |
| [40] |
Z. Sun, Q. Yue, Y. Liu, et al., J. Mater. Chem. A 2 (2014) 18322-18328. DOI:10.1039/C4TA04414B |
| [41] |
X. Fang, C. Chen, Z. Liu, P. Liu, N. Zheng, Nanoscale 3 (2011) 1632-1639. DOI:10.1039/c0nr00893a |
| [42] |
Y.Y. Pu, Y. Li, W. Zhuang, et al., Chin. Chem. Lett. 23 (2012) 1201-1204. DOI:10.1016/j.cclet.2012.07.010 |
| [43] |
W. Li, Y. Deng, Z. Wu, et al., J. Am. Chem. Soc. 133 (2011) 15830-15833. DOI:10.1021/ja2055287 |
| [44] |
J. Liu, J. Cheng, R. Che, et al., ACS Appl. Mater. Interfaces 5 (2013) 2503-2509. DOI:10.1021/am3030432 |
 2020, Vol. 31
2020, Vol. 31 

