b Department of Chemistry, Zhejiang University, Hangzhou 310027, China
Organic solar cells (OSCs) have experienced continuous advancement with the recent evolution of non-fullerene acceptors [1-5]. Especially, the development of acceptor-donor-acceptor (A-D-A) type electron accepting molecules have largely advanced the development of bulk-heterojunction (BHJ) OSCs, including acceptors with fused-ring core, such as ITIC [6], IT-4 F [7], IEICO-4 F [8], ZITI [9], SN6IC-4 F[10], COi8DFIC [11] and IFIC-i-4 F [12, 13], and non-fused core, such as PTIC [14], DF-PCIC [15], HF-TCIC [16], and DFPCBR [17]. These discoveries have instigated the development of a multitude of acceptors with a centrosymmetric structure [18, 19]. Until recently, axisymmetric acceptors, such as Y6 (also called BTP-4 F) have drawn attentions of researchers [16, 20-24]. Instead of A-D-A architecture, Y6 possesses an electron-deficient central core as A-D-A'-D-A structure with V-shape molecular geometry with a dipole moment of 0.87 Debye [25], yielding a remarkable power conversion efficiency (PCE)~16% with voltage loss of about 0.5-0.6 V in solar cells.
Y6 and its analogues [25-32] exhibit characteristics such as a sp3-carbon-free skeleton and narrow bandgap (1.33 eV), which is in contrast to classical FREAs such as IT-4 F with a bandgap of 1.49 eV and skeleton containing sp3-carbon for two up- and downpointing side chains. A centrosymmetric acceptor (SN6IC-4 F) [10] was reported with an optical bandgap of 1.32 eV, which is very similar to the 1.33 eV of Y6. Moreover, SN6IC-4 F possesses sp2-nitrogen replacing sp3-carbons in the fused rings. When combined with PBDB-T, a high PCE of 13.2% was achieved with voltage loss of 0.54 V. To gain in-depth understandings on how structure factors of molecules influence their photovoltaic performance, we therefore propose a comparative study wherein SN6IC-4 F is employed as a bridge between classical centrosymmetric fused acceptors (such as ITIC, IT-4 F) and the V-shape Y6, to sort out their structure-performance correlations. Herein, we present two new electron acceptors, namely SN6-2Br and BTP-2Br, one with a linearand another with a V-shaped core flanked by bromo-modified (2-(3-oxo-2, 3-dihydroinden-1-ylidene) malononitrile (BrIC) [33] to reduce the HOMO-offset between donor and acceptor. The acceptors both possess narrow bandgaps of 1.32 eV and 1.35 eV for SN6-2Br and BTP-2Br, respectively (Fig. 1 and Fig. S1 in Supporting information). Devices based on BTP-2Br exhibit superior devices performances due to an increased exciton dissociation and diffusion efficiency in blend. As a result, PBDB-TF:BTP-2Br based devices achieve a PCE of 13.84% with voltage-loss of only 0.46 V. Furthermore, semitransparent OSCs (ST-OSCs) were fabricated with PCE of 9.62% and average visible transmittance of 20.1% from PBDB-TF:BTP-2Br blends.
|
Download:
|
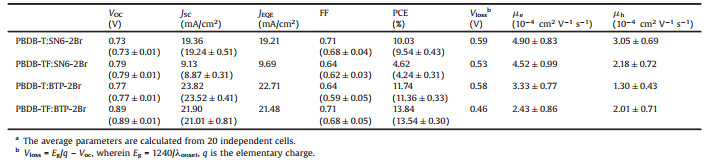
| Fig. 1. (a) Synthetic route of SN6-2Br and BTP-2Br. (b) UV–vis absorption spectra of SN6-2Br and BTP-2Br in solution and neat film. (c) Energy-levels of PBDB-T, PBDB-TF, SN6-2Br and BTP-2Br. | |
The two acceptors (SN6-2Br and BTP-2Br) were obtained through the synthetic routes depicted in Fig. 1a. The intermediates SN6-CHO [10] and BTP-CHO [22] were synthesized according to literature and the target molecules were obtained by Knoevenagel condensations with a yield of 73% and 82% for SN6-2Br and BTP-2Br, respectively. Details on synthesis and structure characterization can be found in Supporting information.
Molecular configurations of SN6-2Br and BTP-2Br were estimated by density functional theory (DFT) calculations with nonlocal density functional of B3LYP with 6-31 G as basis sets. To simplify the calculations, alkyl side chains in both SN6-2Br and BTP-2Br are replaced by methyl groups, except for the 2-ethylhexyl side chains on the nitrogen atoms in BTP-2Br [22]. As seen in Fig. S2 (Supporting information), SN6-2Br keeps a planar geometry while BTP-2Br possesses twisted arms with the dihedral angle of 14.33. The dipole moment of BTP-2Br is 2.80 Debye compared with 0 Debye of SN6-2Br. The large dipole moment in BTP-2Br is caused by the simultaneous influence of the molecular geometry and the intramolecular charge transfer (ICT) effect, which can be referred from the molecular orbital amplitude plots.
The ultraviolet-visible (UV–vis) absorption spectra of SN6-2Br and BTP-2Br in chloroform and as thin films are displayed in Fig. 1b. In solution, the maximum absorption peak is located at 770 nm for SN6-2Br and 732 nm for BTP-2Br, indicating strong ICT effect in the two acceptors. The slightly blue-shifted absorption of BTP-2Br is attributed to the electron-withdrawing properties of the 2, 1, 3-benzothiadiazole (BT) unit. In the neat film, SN6-2Br and BTP-2Br exhibit a maximum absorption peak at 850 nm and 812 nm, respectively, implying an 80 nm red-shift of both compared to the absorptions in solution, which suggests that a strong p-p stacking is present in the film. The optical bandgap of SN6-2Br is 1.32 eV with absorption onset (λonset) of 940 nm, which is slightly smaller than the 1.35 eV of BTP-2Br (λonset of 916 nm).
The energy-levels of SN6-2Br and BTP-2Br were investigated by cyclic voltammetry (CV). The CV plots can be found in Fig. S3 (Supporting information), with the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) levels presented in Fig. 1c. HOMO/LUMO levels of SN6-2Br and BTP-2Br were measured as -5.39/ -3.86 eV and -5.57/ -3.83 eV, respectively. The LUMO-levels of SN6-2Br and BTP-2Br are similar, whilst the HOMO-level of BTP-2Br is downshifted by 0.18 eV compared to SN6-2Br likely due to the presence of BT-unit (Fig. S3). PBDB-T and PBDB-TF were chosen as donor polymers for SN6-2Br and BTP-2Br, respectively.
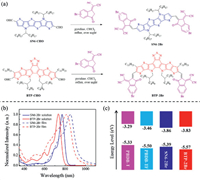
To quantify the photovoltaic properties of these new acceptors, OSCs were fabricated in convention device with the structure of glass/indium tin oxide (ITO)/PEDOT:PSS/BHJ/PFN-Br/Ag, as presented in Fig. 2a. To fully access the performance of the two acceptors, devices were prepared with the active layer systems of PBDB-T:SN6-2Br, PBDB-TF:SN6-2Br, PBDB-T:BTP-2Br and PBDB-TF: BTP-2Br. The corresponding current density–voltage (J–V) curves are showed in Fig. 2b and the device parameters are summarized in Table 1. PBDB-T:SN6-2Br based devices exhibit a PCE of 10.03%, with a JSC of 19.36 mA/cm2, VOC of 0.73 V, and FF of 0.71 (Table 1). Generally, VOC is proportional to the difference between the LUMO of the acceptor and HOMO of the donor (ΔE(LUMOA HOMOD))[34]. The slightly up-shifted LUMO-level of BTP-2Br leads to an increased VOC of 0.77 V when matched with PBDB-T. Meanwhile, JSC is increased to 23.84 mA/cm2, despite the broader bandgap of BTP-2Br. Thereby, devices based on PBDB-T:BTP-2Br achieve an enhanced PCE of 11.72%, the VOC obtained for this active layer combination corresponds to an voltage-loss of 0.58 V (Table 1). The HOMO-offset between PBDB-T and SN6-2Br is only 0.06 eV, which is significantly smaller than the 0.24 eV of PBDB-T and BTP-2Br. In an attempt to test the two acceptors under similar conditions, PBDB-T was changed to PBDB-TF as the HOMO-level aligns much better with BTP-2Br thus achieving an HOMO-offset of 0.07 eV, which is very similar to the offset of 0.06 eV of the PBDB-T:SN6-2Br blended film [34, 35]. The enhanced ΔE(LUMOA HOMOD) endows in devices with an increased VOC herein 0.89 V was obtained. Interestingly, JSC is slightly reduced to 21.90 mA/cm2, which is, however, still larger than the 19.36 mA/cm2 obtained from PBDB-T:BTP-2Br based devices. As a result, the devices achieved a PCE of 13.84%, with the voltage-loss of only 0.46 V (Table 1). To the best of our knowledge, this voltage-loss is one of the lowest values reported for high-performance solar cells (Fig. 2d). While for the PBDB-TF:SN6-2Br based devices with a negative HOMO-offset, a low PCE of only 4.62% was obtained (Table 1).
|
Download:
|
| Fig. 2. (a) OSC device architecture. (b) J–V characteristics under AM 1.5 G illumination (100 mW/cm2) and (c) EQE spectra of the best OSCs. (d) Voltage loss and PCEs for organic solar cells with binary blends in this work and literature. (e) Plot of Jph vs. Veff of the optimized devices. (f) Kinetic traces of ground state bleaching of blend films from fs-TA spectra (excitation wavelength of 750 nm; probe 590 nm for PBDB-T based blends and 610 nm for PBDB-TF based blends). | |
|
|
Table 1 The photovoltaic parameters of the OSCs under the illumination of AM 1.5 G, 100 mW/cm2.a |
External quantum efficiencies (EQEs) of the best performance devices can be seen in Fig. 2c. The calculated current densities (JEQE) from the integration EQE spectra are matched well with those values obtained from J-V curves (Table 1). As expected, the PBDB-T:SN6-2Br based device exhibits a broader photo-response range, the EQE values are, however, lower than 70% throughout the photo-responsive window. In contrast, EQE values of the BTP-2Br based devices are above 70% in the range of 487-819 nm (PBDB-T: BTP-2Br) and 453-819 nm (PBDB-TF:BTP-2Br), resulting in the increased JSC of the BTP-2Br based devices.
Photocurrent density (Jph) versus effective voltage (Veff) were measured to probe the underlying cause of the observed differences in JSC. As seen in Fig. 2e, the saturation photocurrent density (Jsat) varies significantly for the four combinations with values of 20.08 mA/cm2 for PBDB-T:SN6-2Br, 11.05 mA/cm2 for PBDB-TF: SN6-2Br, 24.17 mA/cm2 for PBDB-T:BTP-2Br, and 22.27 mA/cm2 for PBDB-TF:BTP-2Br, which result in a corresponding exciton dissociation probability (Pdiss = Jph/Jsat) of 96.0%, 82.6%, 98.5% and 98.3%, respectively. The larger value of Pdiss for the BTP-2Br-based devices implies an enhanced charge generation, as well as extraction and collection [36], thus increasing the JSC.
The charge carrier mobilities of these BHJs are determined according to the space-charge-limited current (SCLC)-method. The electron mobilities (μe) and hole mobilities (μh) are shown in Table 1. It is evident that SN6-2Br based devices exhibit slightly enhanced μe and μh compared to BTP-2Br based devices, this very slightly improved charge carrier mobilities do, however, not appear to influence the FF as the FF is compatible for devices prepared from both acceptors. All device types exhibit balanced charge carrier mobilities with μe/μh ratios of 1.61, 2.07, 2.57 and 1.21 for PBDB-T/ SN6-2Br, PBDB-TF/SN6-2Br, PBDB-T/BTP-2Br and PBDB-TF/BTP-2Br, respectively. The balanced mobilities in blends contribute to the relatively high FF [37-40], such as PBDB-T:SN6-2Br and PBDB-TF: BTP-2Br based devices.
The charge recombination of devices was investigated via tracking the light intensity (Plight)-dependent J–V characteristics. As shown in Fig. S4 (Supporting information), the slopes for JSC–Plight curves were 0.983 (PBDB-T:SN6-2Br), 0.993 (PBDB-TF:SN6-2Br), 0.989 (PBDB-TF:SN6-2Br) and 0.989 (PBDB-TF:BTP-2Br), suggesting that all devices have good dissociation probabilities at short-circuit condition with slopes close to 1. The dependence of VOC on the Plight reveals that the slopes of devices, are 1.38 (PBDB-T:SN6-2Br), 1.01 (PBDB-TF:SN6-2Br), 1.38 (PBDB-T:BTP-2Br) and 1.12 (PBDB-TF: BTP-2Br) (Fig. S3). Without doubts, for SN6-2Br and BTP-2Br with sp3-carbon-free skeleton, PBDB-T based devices with smaller energetic offset driving force exhibits more severe monomolecular or trap-assisted recombination [34, 41].
Exciton kinetics of blends were further investigated by femtosecond transient absorption (fs-TA) spectroscopy to probe the photoinduced hole transfer dynamics (Figs. 2f, 3a–d and Figs. S5-S7 in Supporting information). The excitation wavelength was 750 nm to excite the acceptors only. Bleach peaks (750 nm and 870 nm in the neat SN6-2Br film, PBDB-T:SN6-2Br film and PBDB-TF:SN6-2Br film) are attributed to the ground state bleach (GSB) and stimulated emission (SE) of the absorption transition in photo-excited SN6-2Br. The decay of the bleach peaks at 870 nm, companied with clear bleach peaks appearing at 590 nm for PBDB-T:SN6-2Br BHJs and 610 nm for PBDB-TF:SN6-2Br BHJs, confirms bi-exponential hole transport from the acceptor to the donors, as these peaks match well with the absorption features of neat polymer donor film. The τ1/τ2 values are 0.51 ps/6.64 ps for PBDB-T/SN6-2Br BHJ and 0.80 ps/ 18.15 ps for PBDB-TF/SN62Br BHJ, with τ1 being assigned to the ultrafast hole transfer from the photoexcited acceptor to the donor and τ2 is the exciton diffusion time in the acceptor towards the interfaces before hole transfer. Similarly, the hole transfer process in BTP-2Br based BHJs were also investigated, in which τ1/τ2 values are 0.37 ps/5.28 ps for PBDB-T/SN6-2Br BHJ and 0.44 ps/5.55 ps for PBDB-TF/SN62Br BHJ. The τ1 of these BTP-2Br based devices are reduced compared to SN6-2Br based devices, indicating a more efficient exciton dissociation. Moreover, the exciton diffusions in BTP-2Br are also more efficient, resulting in the enhanced JSC. Morphology investigations were conducted through atomic force microscopy (AFM) measurements of the film surfaces (Figs. 3e–h). The AFM images of all the films revealed smooth surfaces with the root mean square roughness (RMS) of 2.296 nm (PBDB-T:SN6-2Br), 1.590 nm (PBDB-TF:SN6-2Br), 1.039 nm (PBDB-T:BTP-2Br) and 1.087 nm (PBDB-TF:BTP-2Br).

|
Download:
|
| Fig. 3. Color plot of fs-Transient absorption spectra of (a) PBDB-T: SN6-2Br, (b) PBDB-TF:SN6-2Br, (c) PBDB-T:BTP-2Br and (d) PBDB-TF:BTP-2Br blend film. The AFM of (e) PBDB-T:SN6-2Br, (f) PBDB-TF:SN6-2Br, (g) PBDB-T:BTP-2Br and (h) PBDB-TF:BTP-2Br. The scale bars are 400 nm. | |
Due to the narrow bandgap with portion of visible light transmittance [42, 43] and decent PCE of PBDB-TF:BTP-2Br based devices, we prepared semitransparent organic solar cells (ST-OSC) with an ultra-thin Ag electrode (Fig. 4 and Fig. S8 in Supporting information) [44-46]. With the silver thickness of 10 nm, the devices possess an average visible transmittance (AVT) of 20.1%, CIE coordinates of (0.259, 0.262) and reach a PCE of 9.62%, maintaining 85% of the original JSC. Further increasing the silver thickness to 15 nm results in an increased PCE of 10.82%, a reduction is, however, observed in the AVT to 17.2% (Table S1 in Supporting information). All the JSC values of the ST-OSCs are matched well with the calculated values obtained from EQE curves (Fig. 4b, Table S1).

|
Download:
|
| Fig. 4. (a) J–V curves, (b) EQE curves and (c) visible transmission curves of ST-OSCs with different Ag cathode thicknesses. (d) Color coordinates of ST-OSC incorporating a 10 nm thick Ag layer. | |
In this work, a linear (SN6-2Br) and a V-shaped (BTP-2Br) acceptor were synthesized with similar optical bandgaps and LUMO-levels. The HOMO-level of BTP-2Br had, however, been pushed down by 0.18 eV compared to SN6-2Br. The exciton dissociation was found to be more efficient in the V-shaped BTP-2Br. Besides, the main restriction for PBDB-TF:BTP-2Br device is bimolecular recombination, whereas the monomolecular or trap-assisted recombination constitute a certain proportion in PBDB-T:SN6-Br device, although both the cases are near zero HOMO offset. These differences were also evident in the device performances where PBDB-TF/BTP-2Br BHJ solar cells had a PCE of 13.84%, with a relatively small voltage-loss of 0.46 V, whereas PBDB-T/SN6-2Br only exhibits a performance of 10.03% due to a reduction in VOC and JSC. Semitransparent-OPV were also fabricated and reached a PCE of 9.62% with an AVT of 20.1% at a silver thickness of 10 nm. This work proves the advantages for V-shaped acceptors such as BTP-2Br in efficient exciton dissociation and recombination, thus yielding high-performance OSCs with lower voltage-loss.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research was funded by National Natural Science Founda-tion of China (Nos. 21722404, 21674093 and 21734008), International Science and Technology Cooperation Program of China (ISTCP) (No. 2016YFE0102900), and supported by the Fundamental Research Funds for the Central Universities (No. 2018XZZX002-16). C.-Z. Li thanks the support by Zhejiang Natural Science Fund for Distinguished Young Scholars (No. LR17E030001). Z.-P. Yu thanks the support by the China Postdoctoral Science Foundation Funded Project (No. 2018M632448) and Postdoctoral Science Foundation Funded Project of Zhejiang Province (No. zj2017131).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.12.003.
| [1] |
J. Hou, O. Inganas, R.H. Friend, F. Gao, Nat. Mater. 17 (2018) 119-128. DOI:10.1038/nmat5063 |
| [2] |
P. Cheng, G. Li, X. Zhan, Y. Yang, Nat. Photonics 12 (2018) 131-142. DOI:10.1038/s41566-018-0104-9 |
| [3] |
X. Xu, G. Zhang, Y. Li, Q. Peng, Chin. Chem. Lett. 30 (2019) 809-825. DOI:10.1016/j.cclet.2019.02.030 |
| [4] |
X.W. Zhu, K. Lu, H. Li, R.M. Zhou, Z.X. Wei, Chin. Chem. Lett. 27 (2016) 1271-1276. DOI:10.1016/j.cclet.2016.06.015 |
| [5] |
S. Li, W. Liu, C.Z. Li, M. Shi, H. Chen, Small 13 (2017) 1701120. DOI:10.1002/smll.201701120 |
| [6] |
Y. Lin, J. Wang, Z.G. Zhang, et al., Adv. Mater. 27 (2015) 1170-1174. DOI:10.1002/adma.201404317 |
| [7] |
W. Zhao, S. Li, H. Yao, et al., J. Am. Chem. Soc. 139 (2017) 7148-7151. DOI:10.1021/jacs.7b02677 |
| [8] |
H. Yao, Y. Cui, R. Yu, et al., Angew. Chem. Int. Ed. 56 (2017) 3045-3049. DOI:10.1002/anie.201610944 |
| [9] |
W. Liu, J. Zhang, Z. Zhou, et al., Adv. Mater. 30 (2018) 1800403. DOI:10.1002/adma.201800403 |
| [10] |
C. Huang, X. Liao, K. Gao, et al., Chem. Mater. 30 (2018) 5429-5434. DOI:10.1021/acs.chemmater.8b02276 |
| [11] |
X. Zuo, Jia Xue, Li Dan, et al., Sci. Bull. 62 (2017) 1494-1496. DOI:10.1016/j.scib.2017.10.017 |
| [12] |
F.X. Chen, J.Q. Xu, Z.X. Liu, et al., Adv. Mater. (2018) 1803769. |
| [13] |
Z.X. Liu, T.K. Lau, G. Zhou, et al., Nano Energy 63 (2019) 103807. DOI:10.1016/j.nanoen.2019.06.003 |
| [14] |
Z.P. Yu, Z.X. Liu, F.X. Chen, et al., Nat. Commun. 10 (2019) 2152-2161. DOI:10.1038/s41467-019-10098-z |
| [15] |
S. Li, L. Zhan, F. Liu, et al., Adv. Mater. 30 (2018) 1705208. DOI:10.1002/adma.201705208 |
| [16] |
R. Qin, W. Yang, S. Li, et al., Mater. Chem. Front. 3 (2019) 513-519. DOI:10.1039/C8QM00609A |
| [17] |
N. Wang, W. Yang, S. Li, et al., Chin. Chem. Lett. 30 (2019) 1277-1281. DOI:10.1016/j.cclet.2019.01.010 |
| [18] |
Z. Zhang, W. Liu, T. Rehman, et al., J. Mater. Chem. A 5 (2017) 9649-9654. DOI:10.1039/C7TA01554B |
| [19] |
K. Yan, X. Z.-Liu, X. Li, et al., Org. Chem. Front. 5 (2018) 2845-2851. DOI:10.1039/C8QO00788H |
| [20] |
Z. Yao, X. Liao, K. Gao, et al., J. Am. Chem. Soc. 140 (2018) 2054-2057. DOI:10.1021/jacs.7b13239 |
| [21] |
L. Meng, Y. Zhang, X. Wan, et al., Science 361 (2018) 1094-1100. DOI:10.1126/science.aat2612 |
| [22] |
J. Yuan, Y. Zhang, L. Zhou, et al., Joule 3 (2019) 1140-1152. DOI:10.1016/j.joule.2019.01.004 |
| [23] |
A. Karki, J. Vollbrecht, A.L. Dixon, et al., Adv. Mater. (2019) 1903868. DOI:10.1002/adma.201903868 |
| [24] |
Y. Sun, M. Chang, L. Meng, et al., Nat. Electron. 2 (2019) 513-520. DOI:10.1038/s41928-019-0315-1 |
| [25] |
Y. Cui, H. Yao, J. Zhang, et al., Nat. Commun. 10 (2019) 2515-2523. DOI:10.1038/s41467-019-10351-5 |
| [26] |
R. Yu, H. Yao, Y. Cui, et al., Adv. Mater. (2019) 1902302. |
| [27] |
T. Yan, W. Song, J. Huang, et al., Adv. Mater. 31 (2019) 1902210. DOI:10.1002/adma.201902210 |
| [28] |
X. Xu, K. Feng, Z. Bi, et al., Adv. Mater. 31 (2019) 1901872. DOI:10.1002/adma.201901872 |
| [29] |
B. Fan, D. Zhang, M. Li, et al., Sci. China Chem. 62 (2019) 746-752. DOI:10.1007/s11426-019-9457-5 |
| [30] |
K. Jiang, Q. Wei, J.Y.L. Lai, et al., Joule 3 (2019) 3020-3033. DOI:10.1016/j.joule.2019.09.010 |
| [31] |
J. Yuan, T. Huang, P. Cheng, et al., Nat. Commun. 10 (2019) 570-578. DOI:10.1038/s41467-019-08386-9 |
| [32] |
M. Luo, C. Zhu, J. Yuan, et al., Chin. Chem. Lett. 30 (2019) 2343-2346. DOI:10.1016/j.cclet.2019.07.023 |
| [33] |
Y. Wang, Y. Zhang, N. Qiu, et al., Adv. Energy Mater. 8 (2018) 1702870. DOI:10.1002/aenm.201702870 |
| [34] |
S. Li, L. Zhan, C. Sun, et al., J. Am. Chem. Soc. 141 (2019) 3073-3082. DOI:10.1021/jacs.8b12126 |
| [35] |
C. Yang, J. Zhang, N. Liang, et al., J. Mater. Chem. A 7 (2019) 18889-18897. DOI:10.1039/C9TA04789A |
| [36] |
J. Yuan, Y. Zhang, L. Zhou, et al., Adv. Mater. 31 (2019) e1807577. DOI:10.1002/adma.201807577 |
| [37] |
H. Liu, Z.X. Liu, S. Wang, et al., Adv. Energy Mater. 9 (2019) 1900887. DOI:10.1002/aenm.201900887 |
| [38] |
C.Z. Li, J. Huang, H.X. Ju, et al., Adv. Mater. 28 (2016) 7269-7275. DOI:10.1002/adma.201601161 |
| [39] |
J. Huang, X. Zhang, D. Zheng, et al., Solar RRL 1 (2017) 1600008. DOI:10.1002/solr.201600008 |
| [40] |
K. Yan, C.Z. Li, Macro. Chem. Phys. 220 (2019) 1900084. DOI:10.1002/macp.201900084 |
| [41] |
X. Li, K. Li, D. Su, et al., Chin. Chem. Lett. 31 (2020) 1243-1247. DOI:10.1016/j.cclet.2019.10.029 |
| [42] |
F. Ullah, S. Qian, W. Yang, et al., Chin. Chem. Lett. 28 (2017) 2223-2226. DOI:10.1016/j.cclet.2017.08.009 |
| [43] |
M.N. Shah, S. Zhang, Q. Sun, et al., Tetrahedron Lett. 58 (2017) 2975-2980. DOI:10.1016/j.tetlet.2017.06.056 |
| [44] |
C. Sun, R. Xia, H. Shi, et al., Joule 2 (2018) 1816-1826. DOI:10.1016/j.joule.2018.06.006 |
| [45] |
R. Xia, C.J. Brabec, H.L. Yip, Y. Cao, Joule 3 (2019) 2241-2254. DOI:10.1016/j.joule.2019.06.016 |
| [46] |
X. Li, R. Xia, K. Yan, et al., Chin. Chem. Lett. 31 (2020) 1608-1611. DOI:10.1016/j.cclet.2019.08.046 |
 2020, Vol. 31
2020, Vol. 31