b Media Lab, Massachusetts Institute of Technology, Cambridge, MA 02139, United States;
c Shandong Key Laboratory of Biochemical Analysis, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, China;
d Chemical Sciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, United States
The development of high-performance energy storage devices is of great importance to the advance of eco-friendly and renewable energy [1-7]. Compared with traditional capacitors, supercapacitors not only have the characteristics of high energy and power density, but also have high charge and discharge efficiency, high cycle stability and safety performance. Electric double layer capacitors (EDLCs) are a unique high-power electrochemical energy storage device [8-12]. The storage principle of its capacitance is derived from the charge separation at the electrode/electrolyte interface. Therefore, increasing the porosity and specific surface area of carbon materials is an effective method to enhance their capacitance [13, 14].
Among many electrode materials, carbon materials are the most widely used electrode materials due to their low cost, high conductivity and excellent performance [15]. The application of natural biochar as the electrode material for supercapacitor has become a hot research direction. Many researchers have studied the conversion of different biomass into porous carbon, such as waxberry [2], rice husk [16], tofu [17], pomelo peel [18-20]. Interestingly, the main components of these renewable products are cellulose, hemicellulose, lignin and a small amount of sugar [21]. Precursors with highly heterogeneous and nanoscale periodism are critical to achieving high performance electrode materials [22]. In particular, the grapes are composed of interconnected cellulose network with highly heterogeneous and a large number of glucose units. It can be obtained with high porosity, large specific surface area and stable structure by high temperature carbonization. Cellulose networks are formed by short, multibranched polysaccharide chains and a few interconnects of polyphenolic polymers. During the hydrothermal pre-carbonization process, a large number of glucose units contribute to the increase of oxygen vacancies. The cellulose-rich multiphase structure is favor for honeycomb-like porous carbon (GHPC) through hydrothermal pre-carbonization and chemical activation processes. However, carbon materials belong to hydrophobic materials. The pore accessibility is poor, especially at high scanning rates, resulting in limited power characteristics of porous carbon [23-25]. Hence, in order to increase the capacitance of EDLC, it is still necessary to improve the wettability of carbonaceous material. Heteroatom doping is an effective method [26, 27]. Since the difference in charge between heteroatoms and carbon atoms, heteroatom-doped carbonaceous materials can change the distribution of electron clouds around carbon atoms, and boost the electrochemical performance of carbonaceous materials [28-30]. N-doping can improve the electronic conductivity of carbon and generate more active sites in the carbon skeleton, which has been widely used and achieved the improvement of reactivity in the energy storage system [25, 31, 32].
In this work, a honeycomb-like porous carbon derived from grape was fabricated via a facile method through KOH activation and carbonization. The obtained NGHPC possesses a hierarchically interconnected micro/mesoporous pore structure, and has a high surface area (1268 m2/g) and high content of pyridinic-N (36.29%). These advanced properties are beneficial for excellent supercapacitor performance. Significantly, the NGPHC electrode exhibits a remarkable specific capacitance of 275 F/g at 0.5 A/g in a three-electrode cell. Moreover, the NGHPC//NGHPC symmetric super-capacitor displays a high energy density of 12.6 Wh/kg, and excellent cycling stability of approximately 95.2% capacitance retention after 5000 cycles at 5 A/g. The prominent electrochemical performance of NGHPC was ascribed to high specific surface area, honeycomb-like structure and N-doping, as well as their multiple synergistic effects. Therefore, GHPC exhibits remarkable electrochemical performance with high specific capacitance, high energy density, and excellent cycle stability.
Kyoho grapes were bought from local market. KOH (85%) and NH4Cl (99.5%) were from Sigma-Aldrich. All chemicals were used as received. The grapes with removed the grape peel and seed were firstly washed with deionized water and dried overnight at 105 ℃ in oven. In a typical synthesis, GHPC was prepared by the continuous procedures of KOH activation, pre-carbonization and carbonization of dried grape. The obtained samples were designed as GHPC-T-X, where T represents the pyrolysis temperature, X represents the dosages of KOH (g) with respect to 8 g of dried grape. As an example, the preparation of GHPC-700-1 was shown as follows. Firstly, 8 g of dried grape and 1 g of KOH were mixed with 60 mL of deionized water. The mixed liquid was added to a combustion boat, which was then transferred to a muffle furnace with hydrothermal pre-carbonization at 300 ℃ for 2 h, in order to get more oxygen vacancies and defects exist in the sample. The obtained powder mixture was transferred to a boat in tube furnace after grinded in an agate mortar, followed by carbonized at 700 ℃ for 2 h under N2 atmosphere with a heating rate of 2 ℃/min. Afterwards, GHPC-700-1 was obtained by washed with hydrochloric acid solution (15 wt%) and deionized water until the filtrate became neutral, and then dried at 60 ℃ for 12 h. With this treatment, the transition metals in the samples can be completely removed. Without KOH activation was defined as GHPC-700/ GHPC-800/GHPC-900.
For the synthesis of NGHPC, 1.0 g of GHPC-700-2 and 1.0 g of NH4Cl were mixed into the beaker containing 60 mL of deionized water, and stirred at room temperature for 1 h under ultrasonication. Then the mixture was dried at 60 ℃ for 12 h and further transferred to a boat in tube furnace after grinded in an agate mortar. The mixture was pyrolyzed under N2 at 700 ℃ for 2 h with a ramp rate of 2 ℃/min. The obtained NGHPC was washed with deionized water and dried overnight at 105 ℃ in a vacuum oven.
Characterizations and electrochemical measurements can be found in Supporting information.
The NGHPC was prepared from grape as showed in Fig. S1 (Supporting information). The dried grape was soaked in KOH solution with the hydrothermal pre-carbonization method, which can get more oxygen vacancies and defects exist in the sample [6]. It is obvious that the volume of the grape is expanded after precarbonization and activation. Importantly, the pre-carbonization and activation process with KOH hydrolyzes part of the cellulose, and the grape cell walls destroyed, thereby increasing the grape porosity [13]. Finally, GHPC was obtained by the carbonization. Nitrogen-doping process was conducted to get the NGHPC sample.
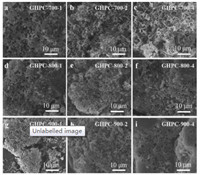
Without activation, all the samples (GHPC-700, GHPC-800 and GHPC-900) presented bulk structures as shown in Fig. S2 (Supporting information). After activation, 3D honeycomb-like porous carbon structure with ordered pores inside can be observed (Fig. 1). During the carbonization process, the alkali metal compounds (K2O and K2CO3) begin to react with carbon, releasing carbon oxide and forming an alkali metal potassium. While potassium passed through the planes of the graphite crystallites, etching occurs on the unexposed surface, thereby increasing the microporous structure and changing the electron distribution [33]. In particular, as the dosage of KOH increases, the pore size gradually increases, and nanosheet structures are formed as shown in Fig. 1 [34, 35]. The increase of KOH dosage led to the increased contact area between KOH and carbon, and the enhanced etching degrees. However, excessive activation led to the reduction of conductivity and specific capacitance of the material [36], because of the poor contact of materials from the larger the pore volume and higher average pore size. Meanwhile, the excessive use of KOH also leads to collapse of the micropores, leading to the poor electrochemical performance of carbon materials. Additionally, with the same dosage of KOH, as the pyrolysis temperature increases, GHPC-700-2, GHPC-800-2, GHPC-900-2 gradually produces more nanosheet structures as shown in Figs. 1b, e and h, which may be related to the degree of graphitization and microtopography [37, 38].
|
Download:
|
| Fig. 1. FESEM images of GHPC samples prepared with various pyrolysis temperatures and different KOH dosages. | |
Figs. 2a and b show the low magnification FESEM images of GHPC-700-2. It can be observed that the material has good porosity and well-developed microstructure. The results can be confirmed from the TEM image as showed in Fig. 2c. The NGHPC was prepared by immersing GHPC-700-2 in NH4Cl solution (detail in the experiment section). The N-doping does not hardly change the microscopic morphology of GHPC-700-2 in Figs. 2d–f. It is indicated that as-obtained NGHPC has good stability and benefits to cycle stability of energy devices. It can be speculated that nitrogen doping of GHPC-700-2 can improve the electrochemical performance, due to the change in electron cloud density around the carbon atom, and the improved wettability between the electrode and the electrolyte [24].

|
Download:
|
| Fig. 2. (a) Low and (b) high magnification FESEM images of GHPC-700-2. (c) The TEM images of GHPC-700-2. (d) Low magnification FESEM images of NGHPC. (e, f) The TEM images of NGHPC. | |
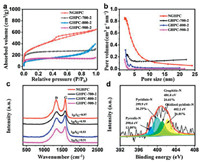
Nitrogen adsorption/desorption measurements were used to investigate the pore structure of GHPC and NGHPC. As shown in Fig. 3a, all the isotherms of GHPC samples belong to the typical type-Ⅰ curve, displaying the existence of microporous structure. In comparison, NGHPC shows a type-Ⅳ isotherm and a H1-type hysteresis loop at a relative pressure P/P0 ranging from 0.45 to 0.85. The hysteresis loop results from filling and emptying of the mesopores by capillary condensation, suggested the generation of a large number of mesopores. This mesoporous structure was proposed to obtain from the N-doping process. Due to the pore structure, NGHPC exhibits a much higher specific area of 1268 m2/g compared to GHPC samples (Table S1 in Supporting information). The specific surface areas of GHPC-700-2, GHPC-800-2 and GHPC-900-2 are calculated to be 986, 656 and 463 m2/g, respectively. Along with the pyrolysis temperature increases, the surface area of GHPC samples gradually decreases, which was attributed to the formation of nanosheet structure as shown in Fig. 1. Table S1 displays the pore characteristics of GHPCs and NGHPC. Compared to GHPC samples, the NGHPC possesses a higher average pore size of 3.06 nm and a higher pore volume of 1.01 cm3/g by the density functional theory method (Fig. 3b). In contract, the average pore size and pore volume of GHPC samples exhibit a trend of increasing and then decreasing along with the activation temperature. As expected, the further introduction of N-doping increases the specific surface area, pore volume and pore size of GHPC-700-2, which could give rise to achieving more defects and active sites and enhance the electrochemical performance.
|
Download:
|
| Fig. 3. (a) Nitrogen adsorption and desorption isotherms and (b) pore size distribution of NGHPC, GHPC-700-2, GHPC-800-2 and GHPC-900-2, respectively. (c) Raman spectra of NGHPC, GHPC-700-2, GHPC-800-2 and GHPC-900-2, respectively. (d) High-resolution N 1s spectrum of NGHPC. | |
Additionally, the crystalline structure of the NGHPC and GHPCs were studied by Raman spectroscopy (Fig. 3c). Obviously, Raman spectra show two characteristic bands for all samples. The D band is located at ~1350 cm-1, which is corresponded to the defects and disordered carbon [38]. The G-band is located at 1580 cm-1, which is attributed to the in-plane vibration of the graphite [39]. It is well known that the intensity ratio of D/G band (ID/IG) indicates the degree of structural disorder with respect to a typical graphitic structure. The NGHPC showed a higher intensity ratio (ID/IG = 0.97) than that of GHPC-700-2 (ID/IG = 0.95), indicating that more defects were induced by nitrogen doping [29, 40]. In comparison, the intensity ratio (ID/IG) of GHPC samples decreases with the temperature increases, which is consistent with previous reports [41]. The greater the degree of graphitization, the greater the degree of ordering of the material, which may result in a decrease performance in EDLCs of the material [41, 42].
The chemical composition of the NGHPC and the chemical status of these elements were determined by XPS measurements. As shown in survey spectra (Fig. S3 in Supporting information), there are three obvious peaks at 284.6, 401, 533 eV, associated with C 1s, N 1s and O 1s peaks. Accordingly, the N content in the NGHPC is about 3.57%. The high-resolution spectrum of N 1s could be further divided into four peaks located at 398.6, 399.9, 401.0 and 402.2 eV corresponded to pyrrolic-N, pyridinic-N, graphitic-N, and oxidized pyidinic-N, respectively. These four types N species are calculated to be 13.09%, 36.29%, 24.61% and 26.01% (Fig. 3d). According to previous reports [43, 44], pyridinic-N and pyrrolic-N located on the edge of the graphite layer generating more active sites to store charges, which significantly improving the pseudocapacitance of carbonaceous material. Graphitic-N can improve the conductivity of carbon materials [23]. Compared with pyrrolic-N and graphitic-N, the higher content of pyridinic-N (36.29%) in NGHPC could induce a large number of defects and more active sites, thus increasing the content of pyridinic-N is the most beneficial method to enhance the capacitance [45]. Consequently, the NGHPC possesses a large specific surface area, abundant porosity and high-content of pyridinic-N, which can remarkably shorten the ion diffusion path and increase the electrode/ electrolyte contact area [46]. Therefore, NGHPC can be used as a promising candidate for supercapacitor electrode material.
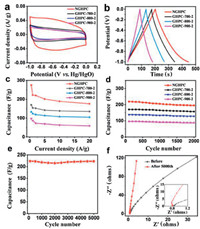
To evaluate supercapacitor performance of GHPCs and NGHPC electrode materials, cyclic voltammetry (CV), galvanostatic charge/ discharge (GCD), and electrochemical impedance spectroscopy (EIS) measurements were performed in a 6 mol/L KOH aqueous solution using a three-electrode system at a scan rate of 100 mV/s. The CV curves of the GHPCs and NGHPC electrodes all exhibit rectangular-like shape (Fig. 4a and Figs. S4–S8 Supporting information), indicating their remarkable capacitive performance. The electrochemical performance (Fig. S4) of GHPC-700, GHPC-800 and GHPC-900 samples were poor, which were attributed the bulky structure (Fig. S2). GHPC-600 presented a much smaller specific capacitance of 120 F/g than that of GHPC-700-2 (175 F/g) at 1 A/g (Figs. S4 and S5). Moreover, the CV curve of NGHPC displays the largest encircled area among all the samples, suggesting its highest capacitance. The specific capacitances of GHPC-700-1/ GHPC-700-4, GHPC-800-1/GHPC-800-4, and GHPC-900-1/GHPC-900-4 were 140/160, 60/95, and 50/63 F/g at 1 A/g, respectively (Figs. S6 and S7). As increasing the pyrolysis temperature, the specific capacitances of GHPC-700-2, GHPC-800-2 and GHPC-900-2 were 175, 130 and 90 F/g at 1 A/g, respectively. When GHPC-700-2 was N-doped (NGHPC), the specific capacitance increased to 222 F/g at 1 A/g, which was 47 F/g higher than the non-N-doped sample (Fig. 4b). The N-doping could improve the wettability of the electrode, induces more electrochemical defects and increases the accumulation of stored charge. Additionally, it was related that nitrogen adsorption and desorption isotherms changed from type Ⅰ of the GHPC to the type Ⅳ of the NGHPC (Fig. 3a). Identically, the specific capacitance of the GHPC and NGHPC was measured at various current densities ranging from 0.5 A/g to 20 A/g. Among the GHPC and NGHPC samples, the GHPC-900-2 electrode exhibited the lowest specific capacitance, which may be caused by the collapse of pores. Notably, the GHPC-700-2, GHPC-800-2, GHPC-900-2 and NGHPC exhibit a specific capacitance of 185, 156, 100 and 275 F/g at a current density of 0.5 A/g (Fig. 4c), respectively. As shown in Table S2 (Supporting information), this specific capacitance of NGHPC is higher than most previously reported carbon-based supercapacitors in the aqueous electrolytes. The cycling stability of the GHPCs and NGHPC was measured at the current density of 1.0 A/g for 2000 cycles (Fig. 4d). As can be seen, the NGPHC electrode reaches a specific capacitance of 214 F/g and exhibits a capacitive retention of about 96% after 2000 cycles, implying the NGHPC electrode has a long-term electrochemical stability. Furthermore, the capacitance of NGPHC maintains an initial capacitance of approximately 99% even after 5000 cycles (Fig. 4e). The outstanding cycling performance was ascribed to the following reason. In the long-term cycle process, the wettability of active material was increased, and the mass diffusion rate was significantly improved. The result was further confirmed by electrochemical impedance spectroscopy (EIS) measurements. Fig. 4f shows the Nyquist plots of NGHPC electrode before and after 5000 cycles. Each Nyquist plot displays a semicircle in high-frequency region and a straight line in low-frequency region. The semicircle represents the charge transfer resistance between the NGHPC electrode and electrolyte. The intercept of the EIS curve at the Z' axis reflects the equivalent series resistance, which consists of the ohmic resistance of the electrolyte, the contact resistance between the NGHPC/current collector and the inherent resistance of the NGHPC. As seen, the NGHPC electrode exhibits a smaller radius of semicircle after 5000 cycles, implying a lower charge transfer resistance. In the intermediate frequency region, the 45 inclined line is derived from ions diffusion rapidly into the electrode. After 5000 cycles, the slope of the line in the low frequency region increased, which was ascribed to the increased wettability after charge-discharge process. Consequently, the NGHPC has low electrochemical resistance, indicating the excellent conductivity.
|
Download:
|
| Fig. 4. (a) CV curves of NGHPC, GHPC-700-2, GHPC-800-2 and GHPC-900-2 electrodes at a scan rate of 100 mV/s in a voltage range of 1.0–0 V. (b) GCD curves at a current density of 1 A/g. (c) Specific capacitance at various current densities of 0.5–20 A/g. (d) Cycling performance of NGHPC, GHPC-700-2, GHPC-800-2 and GHPC-900-2 electrodes at a current density of 1 A/g up to 2000 cycles. (e) Cycling performance of NGHPC electrode up to 5000 cycles at a current density of 1 A/g. (f) The EIS Nyquist plots of NGHPC electrode before and after 5000 cycles. | |
In order to determine the capacitance performance of NGHPC in full cells, symmetric supercapacitors were assembled based on two identical NGHPC electrodes with 6 mol/L KOH aqueous solution as electrolyte. Fig. 5a shows the CV curves of the NGHPC//NGHPC cell at different scan rates. It can be seen that these CV curves have each remained a similar quasi-rectangular shape, even at a high scan rate of 500 mV/s, indicating a quick Ⅰ-Ⅴ response and highly reversible ability. Moreover, the potential window of NGHPC// NGHPC symmetric supercapacitor can be operated up to 1.6 V. The GCD curves at various current densities show a triangular shape and high linearity (Fig. 5b), indicating excellent electrochemical performance. It can be calculated that the specific capacitance of the NGHPC//NGHPC cell at 1.0, 2.0 and 5.0 A/g are 102, 101 and 98 F/g, respectively. To further evaluate the electrochemical performance, a Ragone plot of the NGHPC//NGHPC cell is obtained to describe the relationship between energy density and power density (Fig. 5c). The maximum energy density and power density are 12.6 Wh/kg and 4980 W/kg, which is competitive with previously reported carbon-based symmetric supercapacitors in aqueous solution, such as MSCF [47], ACTs [48], CSs [49], N-HPCNs [50], CN-BC [51], PAN@PANI [52], OMCs [53], NMCs [54]. To further investigate the cycle stability of ASC, a continuous GCD experiment was performed at a current density of 5.0 A/g. As shown in Figs. 5d and e, the NGHPC//NGHPC cell shows a capacitance retention rate of 95.2% after 5000 cycles, indicating that the NGHPC has good stability during charge and discharge process.

|
Download:
|
| Fig. 5. Electrochemical characteristics of NGHPC//NGHPC symmetric supercapa-citor in 6 mol/L KOH aqueous electrolyte in a two-electrode system: (a) CV curves at different scan rates. (b) GCD curves at different current densities. (c) Ragone plots of the energy density and power density between NGHPC and the comparison with other previously reported carbon-based symmetric supercapacitors. (d, e) Cycling stability of NGHPC//NGHPC symmetric supercapacitor after 5000 cycles at 5 A/g. | |
Therefore, NGHPC has excellent electrochemical stability and high specific capacitance, which can be attributed to the following advantages. Firstly, NGHPC possesses a high specific surface area with abundant micropores, which is beneficial for enhancing the charge storage and transportation of electrolyte ions. Secondly, the honeycomb-like structure provides a 3D channel for charge transportation, resulting in rapid charge storage. Finally, N-doping with high-content of pyridinic-N introduces a large number of defects, increases the wettability of the electrode material, and reduces the diffusion resistance of the electrolyte ions. These above results reveal that the NGHPC could be considered as a superior electrode material for high-performance supercapacitors.
In conclusion, as a precursor of activated carbon, the process of transforming grape into porous carbon was be researched. Grapes are good candidates due to highly heterogeneous and nanoscale periodism, availability and low cost. Herein, the grapes are treated in a simple way, including KOH activation, hydrothermal pre-carbonization and carbonization. It was found that the high specific surface area of GHPC could be easily controlled by tuning the weight ratio between grape and KOH. In addition, nitrogen doping significantly improves the wettability of the material and specific surface area, resulting in a significant increase in the specific capacitance. Therefore, we provide a new biomass resource for preparing porous carbon, and the NGHPC is a promising electrode material for supercapacitors.
Declaration of competing interestThe authors declared that they have no conflicts of interest to this work.
AcknowledgmentsThis work is financially supported by the National Natural Science Foundation of China (Nos. 51803093 and 51903123), Natural Science Foundation of Jiangsu Province (Nos. BK20180770 and BK20190760), and Open Project of Chemistry Department of Qingdao University of Science and Technology (No. QUSTHX201921).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.02.020.
| [1] |
H.T. Sun, J. Zhu, D. Baumann, et al., Nat. Rev. Mater. 4 (2019) 45-60. DOI:10.1038/s41578-018-0069-9 |
| [2] |
X. Dong, H. Jin, R. Wang, et al., Adv. Energy Mater. 8 (2018) 1702695. DOI:10.1002/aenm.201702695 |
| [3] |
Z. Liu, L. Jiang, L. Sheng, et al., Adv. Funct. Mate 28 (2018) 1705258. DOI:10.1002/adfm.201705258 |
| [4] |
C. Zheng, Y. Yue, L. Gan, et al., Nanomaterials (Basel) 9 (2019) 937. DOI:10.3390/nano9070937 |
| [5] |
J. Han, H. Wang, Y. Yue, et al., Carbon 149 (2019) 1-18. DOI:10.1016/j.carbon.2019.04.029 |
| [6] |
Q. Wang, Y. Lei, D. Wang, Y. Li, Energy Environ. Sci. 12 (2019) 1730-1750. DOI:10.1039/C8EE03781G |
| [7] |
Z. Huang, L. Li, Y. Wang, et al., Compos. Commun. 8 (2018) 83-91. DOI:10.1016/j.coco.2017.11.005 |
| [8] |
Y. Shao, M.F. El-Kady, J. Sun, et al., Chem. Rev. 118 (2018) 9233-9280. DOI:10.1021/acs.chemrev.8b00252 |
| [9] |
J. Song, W. Shen, J. Wang, W. Fan, Chemelectrochem 5 (2018) 1451-1458. DOI:10.1002/celc.201800305 |
| [10] |
Z. Sun, W. Song, G. Zhao, H. Wang, Cellulose 24 (2017) 4383-4392. DOI:10.1007/s10570-017-1416-5 |
| [11] |
L. Zhang, H. Deng, Q. Fu, Compos. Commun. 8 (2018) 74-82. DOI:10.1016/j.coco.2017.11.004 |
| [12] |
H. Hou, W. Xu, Y. Ding, et al., J. Jiangxi Normal Univ. (Nat. Sci.) 42 (2017) 551-564. |
| [13] |
H. Itoi, H. Nishihara, T. Kogure, T. Kyotani, J. Am. Chem. Soc. 133 (2011) 1165-1167. DOI:10.1021/ja108315p |
| [14] |
M. Jose Mostazo-Lopez, R. Ruiz-Rosas, A. Castro-Muniz, et al., Carbon 129 (2018) 510-519. DOI:10.1016/j.carbon.2017.12.050 |
| [15] |
Y. Wang, Q. Qu, S. Gao, et al., Carbon 155 (2019) 706-726. DOI:10.1016/j.carbon.2019.09.018 |
| [16] |
Y. Fu, N. Zhang, Y. Shen, et al., Bioresour. Technol. 269 (2018) 67-73. DOI:10.1016/j.biortech.2018.08.083 |
| [17] |
L. Yao, J. Yang, P. Zhang, L. Deng, Bioresour. Technol. 256 (2018) 208-215. DOI:10.1016/j.biortech.2018.02.027 |
| [18] |
W. Yuan, Y. Feng, A. Xie, et al., Nanoscale 8 (2016) 8704-8711. DOI:10.1039/C6NR00764C |
| [19] |
N. Wang, T. Li, Y. Song, et al., Carbon 130 (2018) 692-700. DOI:10.1016/j.carbon.2018.01.068 |
| [20] |
H. Liu, Y. Sun, Z. Li, et al., Chin. Chem. Lett. 30 (2019) 1647-1651. DOI:10.1016/j.cclet.2019.06.012 |
| [21] |
M.Q. Zhu, Z.W. Wang, J.L. Wen, et al., Bioresour. Technol. 232 (2017) 159-167. DOI:10.1016/j.biortech.2017.02.033 |
| [22] |
J. Ding, H. Wang, Z. Li, et al., Energy Environ. Sci. 8 (2015) 941-955. DOI:10.1039/C4EE02986K |
| [23] |
Z. Liu, L. Zhang, L. Sheng, et al., Adv. Energy Mater 8 (2018) 1802042. DOI:10.1002/aenm.201802042 |
| [24] |
D. Sun, X.B. Zhu, B. Luo, et al., Adv. Energy Mater. 8 (2018) 1801197. DOI:10.1002/aenm.201801197 |
| [25] |
W. Yang, J. Zhou, S. Wang, et al., Energy Environ. Sci. 12 (2019) 1605-1612. DOI:10.1039/C9EE00536F |
| [26] |
H. Jin, X. Feng, J. Li, et al., Angew. Chem. Int. Ed. 58 (2019) 2397-2401. DOI:10.1002/anie.201813686 |
| [27] |
J. Zhou, J. Lian, L. Hou, et al., Nat. Commun. 6 (2015) 8503. DOI:10.1038/ncomms9503 |
| [28] |
Y. Xie, Y. Chen, L. Liu, et al., Adv. Mater. 29 (2017) 1722268. |
| [29] |
Z. Yuan, J. Li, M. Yang, et al., J. Am. Chem. Soc. 141 (2019) 4972-4979. DOI:10.1021/jacs.9b00154 |
| [30] |
X. Peng, L. Zhang, Z. Chen, et al., Adv. Mater. 31 (20149) 1900341. |
| [31] |
S. Zuo, J. Chen, W. Liu, et al., Carbon 129 (2018) 199-206. DOI:10.1016/j.carbon.2017.12.018 |
| [32] |
Z. Sun, L. Yang, D. Zhang, W. Song, Sensor. Actuator B:Chem. 283 (2019) 579-589. DOI:10.1016/j.snb.2018.12.073 |
| [33] |
S. Zhu, Y. Song, X. Zhao, et al., Nano Res. 8 (2015) 355-381. DOI:10.1007/s12274-014-0644-3 |
| [34] |
M. Sevilla, G.A. Ferrero, N. Diez, A.B. Fuertes, Carbon 131 (2018) 193-200. DOI:10.1016/j.carbon.2018.02.021 |
| [35] |
X. Xi, D. Wu, L. Han, et al., ACS Nano 12 (2018) 5436-5444. DOI:10.1021/acsnano.8b00576 |
| [36] |
O. Barbieri, M. Hahn, A. Herzog, R. Kotz, Carbon 43 (2005) 1303-1310. DOI:10.1016/j.carbon.2005.01.001 |
| [37] |
R. Mo, F. Li, X. Tan, et al., Nat. Commun. 10 (2019) 1474. DOI:10.1038/s41467-019-09274-y |
| [38] |
M. Zheng, S. Zhang, S. Chen, et al., Nano Res. 10 (2017) 4305-4317. DOI:10.1007/s12274-017-1659-3 |
| [39] |
H. He, D. Huang, Y. Tang, et al., Nano Energy 57 (2019) 728-736. DOI:10.1016/j.nanoen.2019.01.009 |
| [40] |
S. Huang, Z. Li, B. Wang, et al., Adv. Funct. Mater. 28 (2018) 1706294. DOI:10.1002/adfm.201706294 |
| [41] |
H. Jiang, J. Gu, X. Zheng, et al., Energy Environ. Sci. 12 (2019) 322-333. DOI:10.1039/C8EE03276A |
| [42] |
S. Jia, Y. Wang, G. Xin, et al., Electrochim. Acta 196 (2016) 527-534. DOI:10.1016/j.electacta.2016.02.196 |
| [43] |
T. Kou, T. Smart, B. Yao, et al., Adv. Energy Mater. 8 (2018) 1703538.. DOI:10.1002/aenm.201703538 |
| [44] |
Z. Peng, Y. Hu, J. Wang, et al., Adv. Energy Mater. 9 (2019) 1802928.. DOI:10.1002/aenm.201802928 |
| [45] |
J. Bai, B. Xi, H. Mao, et al., Adv. Energy Mater. 30 (2018) 1802310. |
| [46] |
J. Gu, Z. Du, C. Zhang, S. Yang, Adv. Energy Mater. 6 (2016) 1600917. DOI:10.1002/aenm.201600917 |
| [47] |
L. Qie, W. Chen, H. Xu, et al., Energy Environ. Sci. 6 (2013) 2497-2504. DOI:10.1039/c3ee41638k |
| [48] |
L.F. Chen, X.D. Zhang, H.W. Liang, et al., ACS Nano 6 (2012) 7092-7102. DOI:10.1021/nn302147s |
| [49] |
L. Bao, X. Li, Adv. Mater. 24 (2012) 3246-3252. DOI:10.1002/adma.201200246 |
| [50] |
K. Sun, Z. Zhang, H. Peng, et al., Mater. Chem. Phys. 218 (2018) 229-238. DOI:10.1016/j.matchemphys.2018.07.052 |
| [51] |
W. Na, J. Jun, J.W. Park, et al., J. Mater. Chem. A 5 (2017) 17379-17387. DOI:10.1039/C7TA04406B |
| [52] |
Z. Song, D. Zhu, D. Xue, et al., ACS Appl. Energy Mater. 1 (2018) 4293-4303. DOI:10.1021/acsaem.8b00928 |
| [53] |
F. Miao, C. Shao, X. Li, et al., J. Mater. Chem. A 4 (2016) 4180-4187. DOI:10.1039/C6TA00015K |
| [54] |
S. Herou, M.C. Ribadeneyra, R. Madhu, et al., Green Chem. 21 (2019) 550-559. DOI:10.1039/C8GC03497D |
 2020, Vol. 31
2020, Vol. 31 

