Persistent organic pollutant wastewater remediation has been of vital significance for realizing a sustainable environmental remediation and natural water resource conservation [1-4]. Among various physical and chemical water treatment techniques dealing with organic pollutants in wastewater, advanced oxidation processes (AOPs) utilizing hydroxyl radicals (·OH) as the oxidant has been intensively investigated in the past decades [5-7]. As one of the most popular AOPs, Fenton reaction has been successfully adopted in various organic pollutants control and recalcitrant compounds degradation [8-14]. However, the required acidic condition is the major drawback for Fenton reactions because of the inevitable neutralization process and secondary sludge pollution generation [10, 15-17]. Therefore, lots of research efforts have been invested into development of effective heterogeneous Fenton catalysts suitable for mild conditions, which could avoid the neutralization process and formation of secondary sludge [18-20]. Various heterogeneous Fenton catalysts including carbon nanotube-supported Fe3O4, nanometer zero valent iron, and metal doped iron oxide have been reported [13, 21-28]. However, these efficient catalysts processing are complicated and expensive, which is not suitable for large-scale applications.
In this report, the low cost mechanochemical method is adopted to fabricate micron size FeS1.92 as heterogeneous Fenton catalysts. The mechanochemical approach exhibits technical advantages of small particle sizes and low agglomeration besides its low cost for large-scale fabrications [29-32]. Specifically, the synthesis of a stable sulfured microscale FeS1.92 by ball milling mackinawite (FeS) with sulfur powder (S) is demonstrated in this work. Mechanochemically fabricated FeS1.92 shows high catalytic activities for heterogeneous Fenton reaction under mild condition. Furthermore, this FeS1.92 Fenton catalyst also shows high sulfide resistance and low Fe3+ leaching.
In mechanical milling synthesis, FeS and S powders were mixed together by different ratios. The ball milling process with intensive fracturing and grinding would promote the solid state reaction of FeS with sulfur, which lead to the formation of FeS1.92 alloy phase [33, 34]. The X-ray diffraction (XRD) pattern of as synthesized sample (Fig. 1a) shows the peaks related to FeS, S and FeS1.92. The peak intensity of S becomes stronger with increasing the molar ratios of S/FeS (Fig. S1 in Supporting information). This XRD results suggest that the mechanochemical synthesis can obtain the FeS1.92 with some FeS and S remained. In order to verify the chemical structures of FeS1.92, we then washed the sample with CS2 to remove the free sulfur. The XRD peaks of washed FeS1.92 exhibit characteristic FeS1.92 peaks. The scanning electron microscope (SEM) image of FeS1.92 sample shows a floccus morphology, which is related to the mixture of free S with FeS (Fig. 1b and Fig. S2 in Supporting information). The elemental mapping shows that the main element components of the as-synthesized catalyst are Fe and S, which are homogeneously distributed (Fig. S3 and Table S2 in Supporting information). The energy dispersive X ray spectros-copy (EDS) measurement suggests the S content in FeS1.92 is about twice than FeS (Table S3 in Supporting information). X-ray photoelectron spectroscopy (XPS) measurement was performed to explore the chemical bond information of the Fe and S. Two main peaks of FeS sample locate at 724.9 eV and 711.4 eV, which can be assigned to 2p1/2 and 2p3/2 of Fe. After sulfuring treatment, these two main peaks shift to the higher banding energy (725.1 eV and 711.6 eV) (Fig. 1c). In contrast, FeS1.92 has two 163.3 eV and 164.4 eV peaks related to S2- and S0 in Fig. 1d. All these results confirm the reaction between FeS and S during the ball-milling process.

|
Download:
|
| Fig. 1. (a) XRD patterns of FeS raw material and obtained FeS1.92 powder before and after washed by CS2. (b) SEM image of FeS1.92. (c) Fe 2p XPS spectra of FeS and FeS1.92. (d) S 2p XPS spectra of FeS and FeS1.92. | |
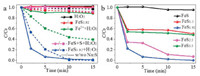
The catalytic activation of H2O2 by FeS1.92 catalyst was tested in the simulated sulfide-containing organic wastewater, which was prepared by adding Na2S in Bisphenol A (BPA) solution under mild conditions. Fig. 2a showed the catalytic activation of H2O2 using different catalysts with or without [Na2S]. Neither FeS1.92 nor H2O2 alone showed any activites for BPA degradation. The regular Fenton system (Fe2+/H2O2) can remove nearly 60% of BPA in the absent of [Na2S]. However, its removal efficiency decreased to only 10% in the presence of [Na2S] because the Fe2+ would be deactivated by S2-. Excitingly, the FeS1.92/H2O2 system exhibited excellent BPA degradation activities with or without [Na2S]. The catalyst of FeS and S, which was prepared by regular mixing and grinding of the same S/FeS molar ratio, did not present the same catalytic performance. Thus, it can be speculated that the alloy of FeS and S was formed by ball-milling, which is important for the heterogeneous Fenton reaction in sulfide-containing organic pollutants water.
|
Download:
|
| Fig. 2. (a) Degradation rates of BPA with FeS1.92 and control experiments. (b). Degradation rates of BPA over several catalysts with different S/FeS molar ratios. Reaction conditions: [catalyst] = 0.25 g/L, [H2O2] = 7.5 mmol/L, [BPA] = 100 mg/L, [Na2S] = 0.2 g/L, pH 6.0. | |
In order to optimize the S/FeS molar ratio for FeSx catalysts, we studied the catalytic activities of several FeSx catalysts with different S/FeS molar ratios. Fig. 2b shows the FeS1.92 show the highest Fenton catalytic performance. It seems that the added S can increase the active sites on the surface of FeS to activate H2O2 to produce ·OH for a better catalytic capacity. When the S powder was insufficient, the catalyst exhibited lower activities. However, too much S would reduce the catalytic activities because too much S will cover the active sites on the surface. The FeS1.92 could be the optimal for FeSx based heterogeneous Fenton catalysts. The pH value and H2O2 concentration have great impact on the Fenton performance of FeS1.92. The degradation efficiency decrease with increase of pH values. The BPA can hardly be oxidized or decomposed when pH values are 8 and 10, which was attributed to the formation of iron hydroxide coating on the catalyst surface and the rapid self-decomposition of H2O2 under alkaline conditions [35, 36]. However, Fig. S4 (Supporting information)shows that a larger amount of Fe3+ leaching was observed under lower pH conditions. The pH 6–7 is the optimal condition to suppress Fe leaching and keep catalytic activity of FeS1.92 (Fig. 3a). Here we selected the pH 6 for the heterogeneous FeS1.92 performance evaluation. When the H2O2 concentration increases from 2.5 mmol/L to 7.5 mmol/L, the degradation efficiency slightly increases (Fig. 3b). Therefore, the H2O2 concentration was maintained at 7.5 mmol/L. Moreover, the S2- concentration in wastewater also affects the degradation performance of FeS1.92. Interestingly, the FeS1.92 showed enhanced catalytic activities for BPA degradation when the S2- concentration reached up to 0.8 g/L (Fig. 3c). Such enhancement might be ascribed to that the more Fe active sites could formed on the FeS1.92 surface under the S2- -rich condition because the Sn2- accumulated on FeS1.92 surface can promote the reduction of Fe3+ to Fe2+ [37]. When the concentration of S2- increased up to 1.2 g/L, it began to inhibit the degradation of BPA and it is due to that the ·OH radicals can react with the redundant S2-, resulting in the inhibition of BPA degradation.

|
Download:
|
| Fig. 3. Effects of pH values (a), H2O2 concentrations (b), Na2S concentrations (c) on BPA removal efficiency. Cyclic stability of FeS1.92/H2O2 system for BPA degradation (d). Reaction conditions: [catalyst] = 0.25 g/L, [BPA] = 100 mg/L, [H2O2] = 7.5 mmol/L for a, c and d, [Na2S] = 0.2 g/L for a, b and d, pH 6.0 for b, c and d. | |
The stability of FeS1.92 was also evaluated under the optimal condition with pH 6.0, 7.5 mmol/L H2O2 and 0.2 g/L Na2S. After four cycles, the FeS1.92 still showed excellent degradation performance and an even better catalytic activity (Fig. 3d). During the cycle experiment, the S2- can be accumulated on FeS1.92 surface, which can promote the Fe3+/Fe2+ cycle and then enhance the performance for the degradation of BPA. Additionally, the Fe3+ concentration in the solution after reaction was below 1.0 ppm in the repeated tests (Fig. S5 in Supporting information). No obvious morphology changes can be found in the FeS1.92 after reaction as shown in SEM image (Fig. S6 in Supporting information). No change is obviously observed in XRD patterns of FeS1.92 before and after the reactions (Fig. S7 in Supporting information), indicating a high chemical stability of FeS1.92. Raman spectroscopy also showed no significant changes on FeS1.92 surface before and after the reaction (Fig. S8 in Supporting information). However, the XPS of FeS1.92 before and after the reaction showed that the proportion of S in FeS1.92 decreased. This is probably because S in FeS1.92 was consumed as a sacrificial agent during the reactions (Fig. 4a and Fig. S9 in Supporting information). These results show that FeS1.92 is a promising catalyst for degradation of sulfide-containing organic pollutants water in heterogeneous Fenton system. We further evaluated the removal efficiency of two more organic pollutants including 2, 4, 6-trichlorophenol (TCP) and sodium benzene sulfonate (SBS). We can see that both TCP and SBS pollutants are completely degraded within 15 min, and their mineralization efficiency was relatively high (Figs. S10 and S11 in Supporting information).
|
Download:
|
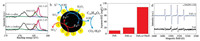
| Fig. 4. (a) The proposed scheme of H2O2 activation by FeS1.92 (b) S 2p XPS spectra of FeS1.92 before and after reaction. (c) The concentration of SO42- in the solution after the reaction. (d) 5, 5-Dimethyl-1-pyrroline N-oxide (DMPO) spin capture EPR spectrum in different reaction systems. Reaction conditions: [catalyst] = 0.25 g/L, [H2O2] = 7.5 mmol/L, [BPA] = 100 mg/L, pH 6.0. | |
The degradation mechanism of organic compounds in FeS1.92/ H2O2 system was investigated. As shown in Fig. 4b, the Fe2+ as the active sites in FeS1.92 was easily oxidized to Fe3+, inducing the formation of the ·OH radicals (Eq.1) [38-41]. Then, the ·OH radicals can mineralize organic pollutants into CO2 and H2O. The S0 or SO42- on FeS1.92 surface, as the electron donor, can react with Fe3+ to form Fe2+ and improve the Fe3+/Fe2+ cycle (Eqs. 2 and 3) [42-44]. Compared with FeS, the chemical bonding between FeS and S in FeS1.92 can promote the Fe3+/Fe2+ cycle for better degradation performance. When the solution contains appropriate amount of S2-, it can also promote the conversion of Fe3+ to Fe2+ (Eq. 4) [37].

|
(1) |

|
(2) |
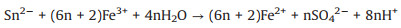
|
(3) |
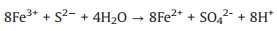
|
(4) |
To verify this speculation, the evolution of surface S in FeS1.92 during Fenton reaction was investigated. According to the XPS analysis, the proportion of S0 or Sn2- in FeS1.92 after the reaction decreased, indicating that S was consumed to some extent in the process of the reaction (Fig. 4a and Table S3 in Supporting information). The sulfur seems to be oxidized into SO42- and can be detected in the final reaction solution (Fig. 4c). Moreover, the SO42- produced in FeS1.92/H2O2 system is about twice as that produced in FeS/H2O2 system, indicating that sulfur added to FeS by ball-milling does participate in the reaction. For a better insight into the role of S in this FeS1.92 Fenton catalyst, the effect of SO42- in the Fenton reaction was investigated. The result indicated that the SO42- did not impact the BPA degradation in FeS1.92/H2O2 system. This result confirmed that the performance enhancement was mainly attributed to the formation of S0 or Sn2- on FeS1.92 surface (Fig. S12 in Supporting information). Electron paramagnetic resonance (EPR) analysis was also conducted to explore the above mechanism. As shown in Fig. 4d, the hydroxyl radical adducts of 5, 5-dimethyl-1-pyrroline N-oxide (DMPO-OH) (1:2:2:1) signal was observed in FeS1.92/H2O2 system, indicating the generation of ·OH radicals in the sulfide-containing solution. The relative EPR intensity of FeS1.92/H2O2 system exhibit higher EPR intensity than that of FeS/H2O2 system. These results indicated that S in FeS1.92 promote the ·OH radicals generation especially in the sulfide-containing organic pollutants water.
In summary, a highly efficient FeS1.92 based heterogeneous Fenton catalyst was synthesized by a facile ball milling of FeS with S powder. In the mechanochemical reaction, the S species can reduce Fe3++ to Fe2+ for Fenton-like reaction. At the same time, this FeS1.92 Fenton catalyst shows a highly sulfide resistance and improved performance for degrading sulfidecontaining organic pollutants. This work has broadened the application of FeSx based environmental catalyst in wastewater treatment, especially for sulfide-containing organic pollutants water.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21777097) and Shanghai Shuguang Grant (No. 17SG11) and the China Postdoctoral Science Foundation (Nos5. 2017M621483, 2018T110397).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.049.
| [1] |
I. Ali, M. Asim, T.A. Khan, J. Environ. Manag. 113 (2012) 170-183. DOI:10.1016/j.jenvman.2012.08.028 |
| [2] |
T. Deblonde, C. Cossu-Leguille, P. Hartemann, Int. J. Hyg. Environ. Health 214 (2011) 442-448. DOI:10.1016/j.ijheh.2011.08.002 |
| [3] |
R. Rosal, A. Rodriguez, J.A. Perdigon-Melon, et al., Water Res. 44 (2010) 578-588. DOI:10.1016/j.watres.2009.07.004 |
| [4] |
J. Xiao, Y. Xie, H. Cao, Chemosphere 121 (2015) 1-17. DOI:10.1016/j.chemosphere.2014.10.072 |
| [5] |
T. An, H. Yang, G. Li, et al., Appl. Catal. B:Environ. 94 (2010) 288-294. DOI:10.1016/j.apcatb.2009.12.002 |
| [6] |
Y. Deng, R. Zhao, Curr. Pollut. Rep. 1 (2015) 167-176. DOI:10.1007/s40726-015-0015-z |
| [7] |
K.E. O'Shea, D.D. Dionysiou, J. Phys. Chem. Lett. 3 (2012) 2112-2113. DOI:10.1021/jz300929x |
| [8] |
A. Babuponnusami, K. Muthukumar, J. Environ. Chem. Eng. 2 (2014) 557-572. DOI:10.1016/j.jece.2013.10.011 |
| [9] |
S. Giannakis, M.I. Polo López, D. Spuhler, et al., Appl. Catal. B:Environ. 199 (2016) 199-223. DOI:10.1016/j.apcatb.2016.06.009 |
| [10] |
E. Neyens, J. Baeyens, J. Hazard. Mater. 98 (2003) 33-50. DOI:10.1016/S0304-3894(02)00282-0 |
| [11] |
M.C. Pereira, L.C.A. Oliveira, E. Murad, Clay Miner. 47 (2018) 285-302. |
| [12] |
J.J. Pignatello, E. Oliveros, A. MacKay, Crit. Rev. Environ. Sci. Technol. 36 (2006) 1-84. DOI:10.1080/10643380500326564 |
| [13] |
S. Rahim Pouran, A.A. Abdul Raman, W.M.A. Wan Daud, J. Clean. Prod. 64 (2014) 24-35. DOI:10.1016/j.jclepro.2013.09.013 |
| [14] |
M. Umar, H.A. Aziz, M.S. Yusoff, Waste Manag. 30 (2010) 2113-2121. DOI:10.1016/j.wasman.2010.07.003 |
| [15] |
A.D. Bokare, W. Choi, J. Hazard. Mater. 275 (2014) 121-135. DOI:10.1016/j.jhazmat.2014.04.054 |
| [16] |
J. Kochany, E. Lipczynska-Kochany, J. Hazard. Mater. 166 (2009) 248-254. DOI:10.1016/j.jhazmat.2008.11.017 |
| [17] |
A. Mirzaei, Z. Chen, F. Haghighat, L. Yerushalmi, Chemosphere 174 (2017) 665-688. DOI:10.1016/j.chemosphere.2017.02.019 |
| [18] |
P.V. Nidheesh, RSC Adv. 5 (2015) 40552-40577. DOI:10.1039/C5RA02023A |
| [19] |
X. Qian, M. Ren, Y. Zhu, et al., Environ. Sci. Technol. 51 (2017) 3993-4000. DOI:10.1021/acs.est.6b06429 |
| [20] |
G. Zhang, S. Wang, F. Yang, J. Phys. Chem. C 116 (2012) 3623-3634. DOI:10.1021/jp210167b |
| [21] |
V. Cleveland, J.P. Bingham, E. Kan, Sep. Purif. Technol. 133 (2014) 388-395. DOI:10.1016/j.seppur.2014.06.061 |
| [22] |
R.A. Crane, T.B. Scott, J. Hazard. Mater. 211- 212 (2012) 112-125. |
| [23] |
J. Deng, X. Wen, Q. Wang, Mater. Res. Bull. 47 (2012) 3369-3376. DOI:10.1016/j.materresbull.2012.07.021 |
| [24] |
I.R. Guimaraes, A. Giroto, L.C.A. Oliveira, et al., Appl. Cata. B:Environ. 91 (2009) 581-586. DOI:10.1016/j.apcatb.2009.06.030 |
| [25] |
B.H. Moon, Y.B. Park, K.H. Park, Desalination 268 (2011) 249-252. DOI:10.1016/j.desal.2010.10.036 |
| [26] |
T. Soltani, A. Tayyebi, B.K. Lee, Appl. Surface Sci. 441 (2018) 853-861. DOI:10.1016/j.apsusc.2018.02.063 |
| [27] |
Y. Wang, H. Zhao, G. Zhao, Appl. Catal. B:Environ. 164 (2015) 396-406. DOI:10.1016/j.apcatb.2014.09.047 |
| [28] |
S. Zha, Y. Cheng, Y. Gao, et al., Chem. Eng. J. 255 (2014) 141-148. DOI:10.1016/j.cej.2014.06.057 |
| [29] |
L.C. Damonte, M.A. Hernández-Fenollosa, B. Marí, J. Alloys. Compd. 434- 435 (2007) 813-815. |
| [30] |
C. Mochales, R.M. Wilson, S.E.P. Dowker, M.P. Ginebra, J. Alloys. Compd. 509 (2011) 7389-7394. DOI:10.1016/j.jallcom.2011.04.033 |
| [31] |
B. Nasiri-Tabrizi, P. Honarmandi, R. Ebrahimi-Kahrizsangi, P. Honarmandi, Mater. Lett. 63 (2009) 543-546. DOI:10.1016/j.matlet.2008.11.030 |
| [32] |
A. Tadjarodi, M. Imani, Mater. Lett. 65 (2011) 1025-1027. DOI:10.1016/j.matlet.2010.12.054 |
| [33] |
P.P. Chin, J. Ding, J.B. Yi, B.H. Liu, J. Alloys. Compd. 390 (2005) 255-260. DOI:10.1016/j.jallcom.2004.07.053 |
| [34] |
C.K. Lin, C.L. Du, G.S. Chen, et al., Mater. Sci. Eng. A 375- 377 (2004) 834-838. |
| [35] |
Y.S. Jung, W.T. Lim, J.Y. Park, Y.H. Kim, Environ. Technol. 30 (2009) 183-190. DOI:10.1080/09593330802468848 |
| [36] |
K. Toda, T. Tanaka, Y. Tsuda, et al., J. Hazard. Mater. 278 (2014) 426-432. DOI:10.1016/j.jhazmat.2014.06.033 |
| [37] |
L. Zhao, Y. Chen, Y. Liu, C. Luo, D. Wu, Chemosphere 188 (2017) 557-566. DOI:10.1016/j.chemosphere.2017.09.019 |
| [38] |
R. Gonzalez-Olmos, F. Holzer, F.D. Kopinke, A. Georgi, Appl. Catal. A:Gen. 398 (2011) 44-53. DOI:10.1016/j.apcata.2011.03.005 |
| [39] |
L. Xu, J. Wang, J. Hazard. Mater. 186 (2011) 256-264. DOI:10.1016/j.jhazmat.2010.10.116 |
| [40] |
L. Xu, J. Wang, Appl. Catal. B:Environ. 123- 124 (2012) 117-126. |
| [41] |
T. Zhou, Y. Li, J. Ji, F.S. Wong, X. Lu, Sep. Purif. Technol. 62 (2008) 551-558. DOI:10.1016/j.seppur.2008.03.008 |
| [42] |
J. Fan, L. Gu, D. Wu, Z. Liu, Chem. Eng. J. 333 (2018) 657-664. DOI:10.1016/j.cej.2017.09.175 |
| [43] |
S. Guo, Z. Yang, Z. Wen, et al., J. Colloid Interface Sci. 532 (2018) 441-448. DOI:10.1016/j.jcis.2018.08.005 |
| [44] |
Y. Zhou, X. Wang, C. Zhu, et al., Water Res. 142 (2018) 208-216. DOI:10.1016/j.watres.2018.06.002 |
 2020, Vol. 31
2020, Vol. 31 

