b College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
Chromium is widely used in electroplating, metal finishing, leather tanning, and other chemical industries [1, 2]. It is a toxic and mobile element mainly existed in the oxidation states of Cr(Ⅲ) and Cr(Ⅵ) [3, 4]. The hexavalent chromium species, chromate (CrO42-, HCrO4-) and dichromate (Cr2O72-), may cause severe problems towards environments and human beings due to its toxicity, carcinogenicity, and fast mobility in soil and aquatic environments [5]. Thus, the sequestration of Cr(Ⅵ) from industrial wastewater is highly desirable.
Various methods, such as adsorption, membrane filtration, ion exchange, reduction, and electrochemical treatment have been reported to remedy Cr(Ⅵ) [6-14]. For example, Li et al. proposed an emerging class of porous material entitled cationic metal-organic frameworks (MOFs) to efficiently remove Cr(Ⅵ) from aqueous solution [15-19]. Luo and his co-workers introduced a powerful MOF+ technique to obtain an extremely high removal capacity (796 mg/g) for chromate [20]. Although these methods showed some promises for Cr(Ⅵ) removal, there are still many inherent drawbacks such as low removal efficiency, poor selectivity, slow kinetics, and high cost. More importantly, most of the reported methods focused on the cases with low concentrations of Cr(Ⅵ), whereas dealing with the wastewater containing high concentrations of Cr(Ⅵ) is still a clear challenge [17, 21]. Therefore, developing a new facile technique for the fast, efficient, and selective removal of Cr(Ⅵ) from the industrial wastewater remains to be a research target.
In this study, a new approach for the efficient removal of Cr(Ⅵ) anions from aqueous solutions was designed based on the selective crystallization of chromate-water clusters using the cationic imine-linked guanidinium ligands. The crystallization method was used mainly because the crystal lattice can exert the structural constraints leading to rejection of the mismatched ions and therefore, obtain exceptional separation selectivity towards Cr(Ⅵ) anions. In addition, when tackling the wastewater containing high concentrations of Cr(Ⅵ), the reactive crystallization method shows a clear advantage over the state of art techniques. Guanidiniumbased compounds with abundant hydrogen donors are found in many biological systems and have been extensively investigated as molecular receptors for various anions [22-25]. In this work, we report the first case for decontamination of chromate by selective crystallization using imine-linked guanidinium ligands.
Condensation of aminoguanidine hydrochloride with bidentate ligands in ethanol yields the cationic 1, 4-benzene-bis(iminoguanidinium) as shown in Fig. S1 (Supporting information). In the preliminary experiments, it is found that if this ligand reacts with chromate anions, a crystalline precipitate forms in the solution, which inspires us to consider this strategy to treat the chromatecontaminated wastewaters. The obtained results indicate that the BBIG-Cl is able to remove Cr(Ⅵ) anions from aqueous solutions in the form of precipitate with a removal efficiency up to 98.70%. The resulting guanidium chromate salts BBIG-CrO4 is extremely insoluble in water (Ksp, BBIG-CrO4 = 8.19 10-9).The excellent Cr(Ⅵ) removal performance of this method can be explained based on the ionic donor-donor acceptor-acceptor (DD AA) hydrogen-bonded complex and the favorable stacking of the planar guanidium cations in the crystal structure [23].
The single-crystal X-ray diffraction analysis performed on the BBIG-CrO4 reveals its crystalline structure which confirms our initial hypothesis. In the structure of BBIG-CrO4 (Fig. 1a), a planar bis(iminoguanidinium) cation crystallizes with a chromate anion in term of 1:1 complex and two water molecules generated from the hydration. The chemical component of a BBIG-CrO4 unit cell is 4 (BBIG(CrO4)(H2O)2). Here two types of BBIG cations can be detected; one is perfectly planar while the other type is slightly bent. These BBIG cations are stacked in an antiparallel fashion in an ABAB pattern in the crystal, with a mean interplanar distance of 3.44 Å (Fig. 1b). Interestingly, the CrO42- anions always appear in pairs in the BBIG-CrO4 crystal. Each CrO42- pair is bridged by 8 OH (water) O(CrO42-)hydrogen bonds (HBs) assisted by 4 water molecules, forming a unique (CrO42-)2(H2O)4 cluster, as shown in Fig. 1c. We performed Density Functional Theory (DFT) calculations [26-34] to investigate the stability of an isolated (CrO42-)2(H2O)4 cluster. If the four water molecules were removed from the cluster, the calculated interaction energy between the two CrO42- anions (EintCr-Cr) is 228.00 kcal/mol, showing strong repulsion. The calculated total binding energy (Ebcluster) of the whole cluster is 88.70 kcal/mol. That indicates that the OH (water)…O(CrO42-) hydrogen bonding interactions and the electrostatic shielding effects of water molecules can greatly weaken the anion-anion repulsion, but not be enough to stabilize an isolated cluster. Hence, it can be concluded that the cationic BBIG ligands play a crucial role in stabilizing (CrO42-)2(H2O)4 clusters in BBIG-CrO4 crystal. Single-crystal X-ray diffraction (XRD) analysis combining with DFT geometry optimization show that, in the BBIG-CrO4 crystal, each (CrO42-)2(H2O)4 cluster is actually encapsulated into a 20-fold NH···O HBs network, including 14 NH···O(CrO42-)and 6 NH···O(H2O) which provided by the surrounding twelve guanidine groups, as shown in Fig. 1d (The details of the hydrogen bonding in the BBIG-CrO4 crystal can be found in Table S3 in Supporting information). For each (CrO42-)2(H2O)4 cluster, such a super-dense HBs network yields a binding energy (EbL-C) of 153.93 kcal/mol. This binding energy is obviously larger than 20 conventional HBs (about 2-5 kcal/mol per one conventional HB), mainly because of the strong electrostatic attractions between the cationic BBIG2+ ligands and the anionic CrO42-. DFT calculations show that the whole electron density surface of the BBIG molecule possesses positive electrostatic potential (ESP), since it has two positive charges formally (Fig. 1e). Moreover, the positive ESPs are mainly distributed around the two guanidine groups and three positive ESP maxima can be found around each guanidine group. Therefore, all the five –NH terminals can be served as HBs donors. On the contrary, the CrO42- molecule has large negative ESPs on its electron density surface (Fig. 1f). Hence, the dense charge-assisted HBs network greatly stabilizes the (CrO42-)2(H2O)4 clusters in the crystal [35, 36]. Note that CrO42- anions are extremely hydrophilic in water with a high solvation energy (958 kJ/mol), and therefore, it is difficult to transfer them to another phase by desolvation [37]. However, owing to the positive charge of the BBIG ligands and tight hydrogen bonds in the BBIG-CrO4 crystal, CrO42- anions are inclined to transfer from aqueous solutions to solid phase by charge-assisted hydrogen bonding interaction.

|
Download:
|
| Fig. 1. (a) The crystal lattice of BBIG-CrO4 determined by single-crystal X-ray diffraction, the hydrogen positions were optimized by density functional theory (DFT) calculations. (b) The stacking patterns of BBIG cations. (c) The structure of the (CrO42-)2(H2O)4 cluster. (d) Schematic diagram of the hydrogen bonds network between one (CrO42-)2(H2O)4 cluster and its surrounding twelve guanidine groups. Electrostatic potential mapping on the electron density van der Waals surfaces (isodensity = 0.001 a.u.) of BBIG (e) and CrO42- (f). | |
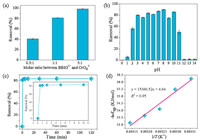
Considering the strong interaction between the CrO42- anions and guanidinium ligands that generates the low soluble BBIG-CrO4 (9.05 10-5mol/L) crystals, the removal performance of Cr(Ⅵ) from aqueous solutions by the ligand was thoroughly investigated. As illustrated in Fig. 2a, the removal efficiency of chromate anions by the BBIG-Cl increases by increasing the equivalent ratio of the BBIG-Cl and chromate anions. The Cr(Ⅵ) removal values are 40.80%, 80.51%, and 98.73% when n = 0.5, 1, and 5 (n is the molar ratio of the ligand and CrO42- anion).
|
Download:
|
| Fig. 2. (a) Removal percentage of CrO42- using the BBIG-Cl ligand with different equivalent ratios of cationic ligands and CrO42-. (b) Effect of pH on the removal of CrO42- by the BBIG-Cl ligand. (c) Removal kinetics of CrO42- by the BBIG-Cl ligand as a function of contact time. (d) Van't Hoff plot for dissolution of BBIG-CrO4 in the range of 25-45 ℃. | |
The maximum removal capacity of Cr(Ⅵ) by the BBIG-Cl is 292.50 mg/g. This value is obviously higher than the determined chromate removal capacities by many other materials (Table S2 in Supporting information). The BBIG-Cl ligand also show a great efficiency in removal of Cr(Ⅵ) from aqueous solution over a wide range of pH values from 2 to 11. However, a slight decrease in the removal efficiency of Cr(Ⅵ) by these ligands is observed at low pH values (Fig. 2b). The observed behaviors of the synthesized ligands in removal of the Cr(Ⅵ) anions from strong acidic to high alkaline solutions are closely relevant with the distribution of Cr(Ⅵ) species in the solution at each pH level [38]. HCrO4- is the predominant form of Cr(Ⅵ) at a low pH value, and has a relatively weaker tendency to attach onto the cationic guanidinium ligands. Fig. 2c shows that within only 30 s, the removal kinetics of CrO42- by the two ligands can reach to the equilibrium. This is the fastest kinetics of the removal of Cr(Ⅵ) from aqueous solutions that has been ever reported (Table S2).
In order to evaluate the selectivity of the introduced crystallization technique, the CrO42- removal experiments using the BBIG-Cl ligand was conducted in presence of different concentrations of NO3- and Cl- anions. In Fig. S5 (Supporting information), the removal percentage of CrO42- in reaction with the BBIG-Cl ligand is 76.74% when m = 1 (m is the NO3- /CrO42- molar ratio). In solutions with the NO3- /CrO42- molar ratio of 10 (m = 10), the Cr(Ⅵ) removal efficiency drops by half. When the value of m reaches up to 100, the BBIG-Cl ligand can only remove 28.50% of the chromate anions from the solution. The removal percentage of chromate by BBIG-Cl is 85.3% when n = 1 (n is the Cl-/CrO42- molar ratio). There is little change in the values of removal percentage when n = 10. BBIG-Cl can still remove 56.86% of chromates from aqueous solution when Cl- anions are 100 times the amount of CrO42- anions. Variable-temperature dissolution measurements of the BBIG-CrO4 revealed that the solubility of these crystals increases with an increase in temperature (Table S1 in Supporting information). The enthalpy of the dissolution obtained from the slope of the Van't Hoff plot (Fig. 2d) is 77.49 kJ/mol for BBIG-CrO4. Thus, the crystallization process of the BBIG-CrO4 is exothermal and entropy driven.
In order to demonstrate the practical application of this new paradigm of chromate separation, a full cycle of chromate removal from an aqueous solution by the BBIG ligand was performed. As illustrated in Scheme 1, CrO42- anions initially get encapsulated by the BBIG ligands and form a solid crystal (Crystal A). In the next step, the BBIG ligand in the isolated crystals can be easily recovered by deprotonation with a 10% NaOH solution. This deprotonation process yields a neutral crystalline BBIG ligand (Crystal B) in 90% yield. In the meantime, about 95% of the CrO42- anions are released into the aqueous solution (Fig. S7 in Supporting information). The formed neutral ligand can then be recycled by their conversion to the cationic form using a diluted HCl solution to be used in the next separation cycle. The overall chromate removal cycle was further confirmed by the PXRD and 1H NMR characterization techniques (Fig. S8 in Supporting information).

|
Download:
|
| Scheme 1. Full separation cycle for CrO42- removal by crystallization using BBIG-Cl ligand. (Ⅰ) CrO42- removal by crystallization with BBIG-Cl. (Ⅱ) Isolation of the BBIG-CrO4 crystals. (Ⅲ) BBIG ligand recovery by neutralization of the BBIG-CrO4 with a NaOH solution; CrO42- anions are released as an aqueous Na2CrO4 solution. (Ⅵ) Cationic BBIG-Cl ligand is regenerated by dissolving neutral BBIG ligand with a diluted HCl solution. | |
In summary, we report the first case of imine-linked guanidinium cationic ligands, namely BBIG2+, for efficiently removing toxic Cr(Ⅵ) oxoanions from aqueous solutions with instantaneous selective crystallization. This work provides a new paradigm for chromate decontamination, wherein the chromate anion is encapsulated as a chromate-water cluster in the solid crystals, which was strongly confirmed by single-crystal X-ray diffraction technique and DFT calculations.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21790374, 21825601, U1732112, 21876124), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Fundamental Research Funds for the Central Universities (No. 2019QNA4047).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.034.
| [1] |
S.A. Saslow, W. Um, C.I. Pearce, et al., Environ. Sci. Technol 52 (2018) 11752-11759. |
| [2] |
J.M. Zachara, C.C. Ainsworth, G.E. Brown, et al., Geochim. Cosmochim. Acta 68 (2004) 13-30. DOI:10.1016/S0016-7037(03)00417-4 |
| [3] |
C.E. Barrera-Diaz, V. Lugo-Lugo, B. Bilyeu, J. Hazard. Mater. 223- 224 (2012) 1-12. |
| [4] |
M. Owlad, M.K. Aroua, W.A.W. Daud, et al., Water Air Soil Pollut. 200 (2008) 59-77. |
| [5] |
M. Panda, A. Bhowal, S. Datta, Environ. Sci. Technol. 45 (2011) 8460-8466. DOI:10.1021/es2015346 |
| [6] |
J. Li, X. Wang, G. Zhao, et al., Chem. Soc. Rev. 47 (2018) 2322-2356. DOI:10.1039/C7CS00543A |
| [7] |
J. Li, X. Zhang, M. Liu, et al., Environ. Sci. Technol. 52 (2018) 2988-2997. DOI:10.1021/acs.est.7b06502 |
| [8] |
L. Li, X.Q. Feng, R.P. Han, J. Hazard. Mater. 321 (2017) 622-628. DOI:10.1016/j.jhazmat.2016.09.029 |
| [9] |
M. Chen, Y. Wu, C.T. Jafvert, Environ. Sci:Nano 4 (2017) 1534-1543. DOI:10.1039/C7EN00382J |
| [10] |
S. Jansone-Popova, A. Moinel, J.A. Schott, et al., Environ. Sci. Technol. 53 (2019) 878-883. DOI:10.1021/acs.est.8b04215 |
| [11] |
B. Ranjan, S. Pillai, K. Permaul, et al., J. Hazard. Mater. 363 (2019) 73-80. DOI:10.1016/j.jhazmat.2018.07.116 |
| [12] |
J.X. Shi, B.G. Zhang, R. Qiu, et al., Environ. Sci. Technol. 53 (2019) 3198-3207. DOI:10.1021/acs.est.8b05053 |
| [13] |
B.G. Zhang, C.P. Feng, J.R. Ni, et al., J. Power Sources 204 (2012) 34-39. DOI:10.1016/j.jpowsour.2012.01.013 |
| [14] |
H. Dong, J. Deng, Y. Xie, et al., J. Hazard. Mater. 332 (2017) 79-86. DOI:10.1016/j.jhazmat.2017.03.002 |
| [15] |
L. Khezami, R. Capart, J. Hazard. Mater. 123 (2005) 223-231. DOI:10.1016/j.jhazmat.2005.04.012 |
| [16] |
K. Li, P. Li, J. Cai, et al., Chemosphere 154 (2016) 310-318. DOI:10.1016/j.chemosphere.2016.03.100 |
| [17] |
S. Rapti, D. Sarma, S.A. Diamantis, et al., J. Mater. Chem. A 5 (2017) 14707-14719. DOI:10.1039/C7TA04496H |
| [18] |
A.V. Desai, B. Manna, A. Karmakar, Angew. Chem. Int. Ed. 55 (2016) 7811-7815. DOI:10.1002/anie.201600185 |
| [19] |
M.B. Luo, Y.Y. Xiong, H.Q. Wu, et al., Angew. Chem. Int. Ed. 56 (2017) 16376-16379. DOI:10.1002/anie.201709197 |
| [20] |
H. Yang, H. Fei, Chem. Commun. 53 (2017) 7064-7067. DOI:10.1039/C7CC04375A |
| [21] |
M. Li, S. Schlesiger, S.K. Knauer, C. Schmuck, Angew. Chem. Int. Ed. 54 (2015) 2941-2944. DOI:10.1002/anie.201410429 |
| [22] |
R. Custelcean, Chem. Commun. 49 (2013) 2173-2182. DOI:10.1039/c2cc38252k |
| [23] |
R. Custelcean, N.J. Williams, C.A. Seipp, Angew. Chem. Int. Ed. 54 (2015) 10525-10529. DOI:10.1002/anie.201506314 |
| [24] |
R. Custelcean, N.J. Williams, C.A. Seipp, et al., Chem.-Eur. J. 22 (2016) 1997-2003. DOI:10.1002/chem.201504651 |
| [25] |
C. Xiao, A. Khayambashi, S. Wang, Chem. Mater. 31 (2019) 3863-3877. DOI:10.1021/acs.chemmater.9b00329 |
| [26] |
J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77 (1996) 3865-3868. DOI:10.1103/PhysRevLett.77.3865 |
| [27] |
B. Dellry, J. Chem. Phys. 92 (1990) 508-517. DOI:10.1063/1.458452 |
| [28] |
B. Dellry, J. Chem. Phys. 113 (2000) 7756-7764. DOI:10.1063/1.1316015 |
| [29] |
A.D. Becke, J. Chem. Phys. 98 (1993) 5648-5652. DOI:10.1063/1.464913 |
| [30] |
W.J. Hehre, W.A. Lathan, J. Chem. Phys. 56 (1972) 5255-5257. DOI:10.1063/1.1677028 |
| [31] |
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B:Condens. Matter 37 (1988) 785-789. DOI:10.1103/PhysRevB.37.785 |
| [32] |
M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian 09, Revision E.01, Gaussian Inc., Wallingford, 2009.
|
| [33] |
T. Lu, F. Chen, J. Comput. Chem. 33 (2012) 580-592. DOI:10.1002/jcc.22885 |
| [34] |
H. Fei, M.R. Bresler, S.R. Oliver, J. Am. Chem. Soc. 133 (2011) 11110-11113. DOI:10.1021/ja204577p |
| [35] |
R. Custelcean, A. Bock, B.A. Moyer, J. Am. Chem. Soc. 132 (2010) 7177-7185. DOI:10.1021/ja101354r |
| [36] |
S. Kubik, Chem. Soc. Rev. 39 (2010) 3648-3663. DOI:10.1039/b926166b |
| [37] |
J.Y. Tong, E.L. King, J. Am. Chem. Soc. 75 (1953) 6180-6186. DOI:10.1021/ja01120a022 |
| [38] |
A.K. Sengupta, D. Clifford, S. Subramonian, Water Res. 20 (1986) 1177-1184. DOI:10.1016/0043-1354(86)90064-3 |
 2020, Vol. 31
2020, Vol. 31 

