b University of Chinese Academy of Sciences, Beijing 100085, China;
c Laboratory of Environmental Nanotechnology and Health Effect, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China;
d Department of Chemistry and Biochemistry, Florida International University, Miami, FL 33199, United States;
e Institute of Environment and Health, Jianghan University, Wuhan, 430056, China
Gold (Au), as a precious metal, was utilized 6000 years ago in ancient Egypt [1]. In recent decades, the demand of Au further increases due to the growing electronic and biomedical applications. Although the average concentration of Au in Earth's crust is relatively low (~1.3 μg/kg) [2], Au can be significantly accumulated in deposits [3]. The supergene transport of Au in surficial environments plays a crucial role in the redistribution of Au from primary sources and the formation of secondary deposits. It has been estimated that one fourth of the mined Au is derived from secondary deposits, following a dissolution-precipitation process of primary Au [4]. The exploration of secondary Au deposits calls for an in-depth understanding of the transport process and mechanism of Au in surficial environments.
It has been recently demonstrated that cyanide (CN-) secretion from a variety of bacteria can accelerate the oxidative dissolution of metallic Au [5, 6] by forming dissolved Au(CN)2-. Other biogenic and abiogenic complexing reagents, e.g., amino acid, thiol, thiosulphate, and halogen, can also dissolve metallic Au into various soluble complexes [6-8]. For example, in pyrite oxidation process, the resulting thiosulphate can induce the oxidative dissolution by forming Au(S2O3)23- [9]. Therefore, in tailing and natural surface waters, soluble Au(Ⅰ/Ⅲ)-complexes (e.g., Au(CN)2-, mixed hydroxide-halide complex) were identified as important oxidation states and complexation forms of Au in aqueous systems [10]. These soluble Au complexes can be further transformed into metallic Au nanoparticles (AuNPs) under biological or chemical reaction. A variety of plants [11-14] and microorganisms [15-19] can reduce Au(Ⅰ/Ⅲ)-complexes into AuNPs. It is believed that bacterial biofilms play a key role in the biomineralization of secondary Au [20-22]. Recently, it was also discovered that dissolved organic matter (DOM) in environmental waters can reduce AuCl4- into AuNPs under sunlight or thermal irradiation [23-25]. Similarly, extracellular polymeric substances can mediate the reduction of AuCl4- into AuNPs [26]. This agrees well with that carbonaceous rocks are the major host rocks of the gold mineralization in some Au deposits [27-29]. However, whether and how these AuNPs further transform into visible Au granules is still not well known and relevant knowledge will enhance our understanding of mobility and supergene enrichment of Au.
In this work, we firstly prepared AuNPs solution from sunlightmediated reduction by DOM. The slow (in years) growth of these AuNPs into visible Au granules was further observed. Morphology characterizations were performed to elucidate the possible growth mechanism of these Au granules. This study will be helpful in understanding the supergene mineralization and transport of secondary Au.
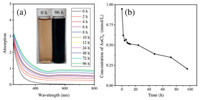
The photochemical formation of AuNPs was investigated at pH 5.8 by using humic acid as a natural reductant. This acidic pH is general in the environment of gold mining-induced acid mine tailings [30]. In addition, AuCl4 occurs in these environments with relatively high concentration [10]. Under natural sunlight irradiation (photosynthetically active radiation was given in Fig. S1 in Supporting information), the color of the solution turned from yellow to blue-black. The evolution of the UV–vis spectrum of this solution is shown in Fig. 1a. An almost-flat absorption curve with a broad peak is observed, with a weak surface plasmon resonance (SPR) of AuNPs at ~600 nm. The flat absorption curve suggested that the formed AuNPs were highly aggregated or polydispersed both in size and shape [31]. With the increasing irradiation time, the UV–vis absorption intensity increased, indicating the continual formation and growth of AuNPs. Then, centrifugal ultrafiltrationinductively coupled plasma-mass spectrometry (ICP-MS) analysis was used to quantify the conversion of AuCl4- to AuNPs. As showed in Fig. 1b, with irradiation, the concentration of ionic Au drastically decreased in the first 4 h, followed by a slow decrease for the rest of time. The changes of ionic Au concentration demonstrated the continual reduction of AuCl4- and formation of AuNPs. The recovery of AuCl4- in the presence of DOM (0 h in Fig. 1b) was 94.6%, demonstrating the complexation of DOM with ionic Au(Ⅲ) is neglectable in the centrifugal ultrafiltration process. In addition, a significant decrease of pH from 5.8 to 4.5 was observed in the reduction process, which agrees with our previous study [24].
|
Download:
|
| Fig. 1. Evolution of (a) UV–vis spectra and (b) concentration of ionic Au in AuCl4- -DOM solution under sunlight. Conditi ons: 5 mg C/L Aldrich humic acid, 1 mmol/L HAuCl4, pH 5.80, natural sunlight irradiation and air temperature (25-37 ℃). | |
The transmission electron microscopy (TEM) images and energy dispersive X-ray spectroscopy (EDS) (Fig. 2) were used to validate the formation of AuNPs. Besides spherical nanoparticles, the branched nanocrystals including bipod and tripod were also observed. The high resolution TEM images (Figs. 2b, e and h) showed that the distances between two adjacent planes in nanoparticle are mainly 0.236 and 0.204 nm, corresponding to the Au(111) and Au(200) lattice planes. The EDS spectra (Figs. 2c, f and i) further confirmed that the particles in TEM are Au-containing nanoparticles. Under sunlight irradiation, firstly generated spherical AuNPs play as seeds for further particle growth. The high energy due to large surface-to-volume ratio and high collision frequency contribute to great mobility of these initially small AuNPs and drive their attachment [32]. The branched nanocrystals are likely to be formed by the further reduction of AuCl4- and attachment-based growth of adjacent spherical AuNPs [32, 33]. According to our previous study [24], in the photochemical reduction process, DOM serves as not only the reductive agent, but also the coating agent to stabilize and disperse AuNPs. Phenolic, alcoholic, and aldehyde groups in DOM act as reductive sites and Au(Ⅰ) is an important intermediate in this reductive process [24].

|
Download:
|
| Fig. 2. TEM images and EDS of the AuNPs formed under different incubation time. (a–c) 4 h; (d–f) 24 h; (g–i) 96 h. | |
Fig. S2 (Supporting information) shows the X-ray diffraction (XRD) pattern of the AuNPs formed in DOM solution. Four diffraction peaks corresponding to the (111), (200), (220), and (311) planes of a face-centered cubic lattice of Au were observed. It should be noted that the peak diffraction corresponding to (111) plane was more intense than peaks from other planes. The intensity ratio between (200) and (111) diffraction peaks (0.25) was much lower than conventional value (0.52) [34], demonstrating that the (111) plane is the predominant orientation. This agrees well with previous study that the growth of the branched nanostructure occurs preferentially on (111) planes [34]. Considering that the multipods typically exhibit twin boundaries that originate from the center of a nanoparticle, the multipods are likely to be formed by reduction of AuCl4- over the (111) plane adjacent to the twin boundaries in the growth process [33]. Thus, the ratio of (111) plane should increase in the formation of multipods or branched nano-structures.
Then the AuNPs formed in DOM solution were stored in a dark environment at ambient temperature to investigate their further transformation. After 4.0 years, visible yellow Au granules can be observed in the blue-black AuNPs solution (Fig. 3a), demonstrating the further growth and fusion of AuNPs into bigger Au granules. After another 2.8 years, the blue-black solution turned to transparent and more Au granules can be observed (Fig. 3b). These Au granules were easy to settle and agglomerate into loosely large aggregates (Fig. 3b), which is favorable for their growth into Au grains. Accordingly, the solution pH further decreased from 4.5 (newly prepared AuNPs) to 4.2 during the 6.8 years storage.

|
Download:
|
| Fig. 3. Photographs showing the formation of Au granules after (a) 4.0 years, and (b) 6.8 years. | |
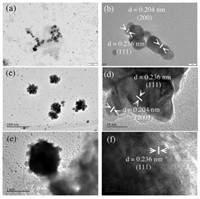
We further used TEM and optical microscope to characterize the morphology of these Au granules. As shown in Figs. 4a and b, similar to the fresh prepared AuNPs solution, spherical nanoparticles and multipods can still be observed after 6.8 years. Interestingly, Au nanoflowers with size ~40 nm were also observed (Figs. 4c and d). In addition, as shown in Figs. 4e and f, these nanoflowers further attached onto a micron-sized Au particle and grew into a "meatball"-like structure [35]. The Au nanoflowers were formed by the selective fusion of large numbers spherical AuNPs to specific crystal faces (possibly (111) face) of the initial small nanoparticles under limited DOM concentration [36]. Under dark condition, the slow reduction of AuCl4- and growth of Au atom is favorable for the anisotropic growth and formation of Au nanoflowers [37]. Optical microscopy was then used to image the visible Au granules. As shown in Fig. S3 (Supporting information), besides needle-like structure (in the centre of Fig. S3d), dendritic structures could be observed universally. These dendritic branches may be assembled from the needle crystals [38]. It should be noted that these dendritic structures are also widely observed in naturally occurring Au granules (Fig. S4 in Supporting information), indicating their similar formation processes. According to previous study, a flower-like structure demonstrates that the assembly or growth kinetics is reaction limited, while diffusion-limited aggregation results in fractal dendrite-like structure [36]. The co-occurrence of flower-and dendrite-like structures suggests a reaction- and diffusion-limited processes coexist in the solution.
|
Download:
|
| Fig. 4. TEM of different sized Au particles in Au granules solution, (a, b), (c, d), and (e, f) show the spherical nanoparticles and multipods, Au nanoflowers, and "meatball"-like Au particles, respectively. | |
Similar to AuNPs formed in DOM solution, the XRD pattern of Au granules also showed a characteristic of face-centered cubic lattice of Au [39], with (111) as the dominate plane (Fig. S2). The intensity ratio between (200) and (111) diffraction peaks (0.23) further decreased, compared with that of fresh AuNPs formed in DOM solution (0.25), indicating the growth of Au atom along the (111) planes.
The ionic Au in Au granule solution detected by centrifugal ultrafiltration-ICP-MS is much higher (0.56 mmol/L) than that in AuNP solution after 96 h (0.21 mmol/L). The changes of ionic Au concentration and morphology indicated that the initial AuNPs formed under sunlight subjected to further dissolution and reduction process under dark condition.
In summary, for the first time, we demonstrated that sunlightinduced AuNPs by DOM can further grow into visible Au granules under ambient environmental conditions. Morphology analysis showed these Au granules possess flower or fractal dendrite-like branched gold structures. This growth process of AuNPs into visible Au granules may play a critical role in the supergene mineraliza-tion and enrichment of secondary Au and therefore drives the biogeochemical cycle of Au.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank for financial supports from the National Natural Science Foundation of China (No. 21777178), Key Projects for Frontier Sciences of the Chinese Academy of Sciences (No. QYZDB-SSWDQC018), and the CAS Interdisciplinary Innovation Team (No. JCTD-2018-04). Y. Yin acknowledges supports from the National Young Top-Notch Talents (No. W03070030) and Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 2016037).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.11.030.
| [1] |
D. Klemm, R. Klemm, A. Murr, J. Afr. Earth Sci. 33 (2001) 643-659. DOI:10.1016/S0899-5362(01)00094-X |
| [2] |
I.K. Pitcairn, Tran. Inst. Min. Metall. (Sect. B:App. Earth Sci.) 120 (2011) 31-38. DOI:10.1179/1743275811Y.0000000021 |
| [3] |
J. Shuster, F. Reith, Minerals 8 (2018) 401. DOI:10.3390/min8090401 |
| [4] |
H.E. Frimmel, Earth Planet. Sci. Lett. 267 (2008) 45-55. DOI:10.1016/j.epsl.2007.11.022 |
| [5] |
A. Avellan, M. Simonin, E. McGivney, et al., Nat. Nanotechnol. 13 (2018) 1072-1077. DOI:10.1038/s41565-018-0231-y |
| [6] |
E. McGivney, X. Gao, Y. Liu, et al., Environ. Sci. Technol. 53 (2019) 1287-1295. DOI:10.1021/acs.est.8b05884 |
| [7] |
M.G. Aylmore, D.M. Muir, Miner. Eng. 14 (2001) 135-174. DOI:10.1016/S0892-6875(00)00172-2 |
| [8] |
S. Praharaj, S. Panigrahi, S. Basu, et al., J. Photochem. Photobio. A:Chem. 187 (2007) 196-201. DOI:10.1016/j.jphotochem.2006.10.019 |
| [9] |
M. Melashvili, C. Fleming, I. Dymov, D. Matthews, D. Dreisinger, Miner. Eng. 87 (2016) 2-9. DOI:10.1016/j.mineng.2015.07.017 |
| [10] |
C. Ta, F. Reith, J. Brugger, A. Pring, C.E. Lenehan, Environ. Sci. Technol. 48 (2014) 5737-5744. DOI:10.1021/es404919a |
| [11] |
C.W.N. Anderson, R.R. Brooks, R.B. Stewart, R. Simcock, Nature 395 (1998) 553-554. DOI:10.1038/26875 |
| [12] |
R.G. Haverkamp, A.T. Marshall, D. van Agterveld, J. Nanopart. Res. 9 (2007) 697-700. DOI:10.1007/s11051-006-9198-y |
| [13] |
I.R. Beattie, R.G. Haverkamp, Metallomics 3 (2011) 628-632. DOI:10.1039/c1mt00044f |
| [14] |
M. Lintern, R. Anand, C. Ryan, D. Paterson, Nat. Comm. 4 (2013) 2614. DOI:10.1038/ncomms3614 |
| [15] |
A. Ahmad, S. Senapati, M.I. Khan, et al., Nanotechnology 14 (2003) 824-828. DOI:10.1088/0957-4484/14/7/323 |
| [16] |
S. He, Z. Guo, Y. Zhang, et al., Mater. Lett. 61 (2007) 3984-3987. DOI:10.1016/j.matlet.2007.01.018 |
| [17] |
M.I. Husseiny, M.A. El-Aziz, Y. Badr, M.A. Mahmoud, Spectrochim. Acta A 67 (2007) 1003-1006. DOI:10.1016/j.saa.2006.09.028 |
| [18] |
A. Ahmad, P. Mukherjee, S. Senapati, et al., Colloid. Surf. B 28 (2003) 313-318. DOI:10.1016/S0927-7765(02)00174-1 |
| [19] |
M. Lengke, G. Southam, Geochim. Cosmochim. Acta 70 (2006) 3646-3661. DOI:10.1016/j.gca.2006.04.018 |
| [20] |
M.A. Rea, C.M. Zammit, F. Reith, FEMS Microbiol. Ecol. 92 (2016) fiw082. DOI:10.1093/femsec/fiw082 |
| [21] |
F. Reith, L. Fairbrother, G. Nolze, et al., Geology 38 (2010) 843-846. DOI:10.1130/G31052.1 |
| [22] |
M.A. Rea, C.D. Standish, J. Shuster, A. Bissett, F. Reith, FEMS Microbiol. Ecol. 94 (2018) fiy080. |
| [23] |
Y. Yin, J. Liu, G. Jiang, ACS Nano 6 (2012) 7910-7919. DOI:10.1021/nn302293r |
| [24] |
Y. Yin, S. Yu, J. Liu, G. Jiang, Environ. Sci. Technol. 48 (2014) 2671-2679. DOI:10.1021/es404195r |
| [25] |
T.E.G. Alivio, N.A. Fleer, J. Singh, et al., Environ. Sci. Technol. 52 (2018) 7269-7278. DOI:10.1021/acs.est.8b01003 |
| [26] |
F. Kang, X. Qu, P.J.J. Alvarez, D. Zhu, Environ. Sci. Technol. 51 (2017) 2776-2785. DOI:10.1021/acs.est.6b05930 |
| [27] |
R.R. Large, S.W. Bull, V.V. Maslennikov, Econ. Geol. 106 (2011) 331-358. DOI:10.2113/econgeo.106.3.331 |
| [28] |
P.R. Sahoo, A.S. Venkatesh, J. Earth Syst.Sci. 123 (2014) 1693-1703. DOI:10.1007/s12040-014-0488-y |
| [29] |
F. Reith, L. Stewart, S.A. Wakelin, Chem. Geol. 320 (2012) 32-45. |
| [30] |
A. Akcil, S. Koldas, J. Clean. Prod. 14 (2006) 1139-1145. DOI:10.1016/j.jclepro.2004.09.006 |
| [31] |
L. Wang, G. Wei, C. Guo, et al., Colloid. Surf. A 312 (2008) 148-153. DOI:10.1016/j.colsurfa.2007.06.043 |
| [32] |
B. Lim, Y. Xia, Angew. Chem. Int. Ed. 50 (2011) 76-85. DOI:10.1002/anie.201002024 |
| [33] |
C.H. Kuo, M.H. Huang, Langmuir 21 (2005) 2012-2016. DOI:10.1021/la0476332 |
| [34] |
B.K. Jena, C.R. Raj, Langmuir 23 (2007) 4064-4070. DOI:10.1021/la063243z |
| [35] |
J. Fang, S. Du, S. Lebedkin, et al., Nano Lett. 10 (2010) 5006-5013. DOI:10.1021/nl103161q |
| [36] |
S.K. Das, C. Dickinson, F. Lafir, D.F. Brougham, E. Marsili, Green Chem. 14 (2012) 1322-1334. DOI:10.1039/c2gc16676c |
| [37] |
Z. Wang, Z. Feng, L. Lin, P. Huang, Z. Zheng, Appl. Surf. Sci. 356 (2015) 1314-1319. DOI:10.1016/j.apsusc.2015.08.234 |
| [38] |
J.H. Lee, K. Kamada, N. Enomoto, J. Hojo, Chem. Lett. 36 (2007) 728-729. DOI:10.1246/cl.2007.728 |
| [39] |
S. Liu, X.L. Zhou, M.M. Zhang, et al., Chin. Chem. Lett. 27 (2016) 843-846. DOI:10.1016/j.cclet.2016.01.019 |
 2020, Vol. 31
2020, Vol. 31 

